Nucleophilicity of EtSH and MeNH2
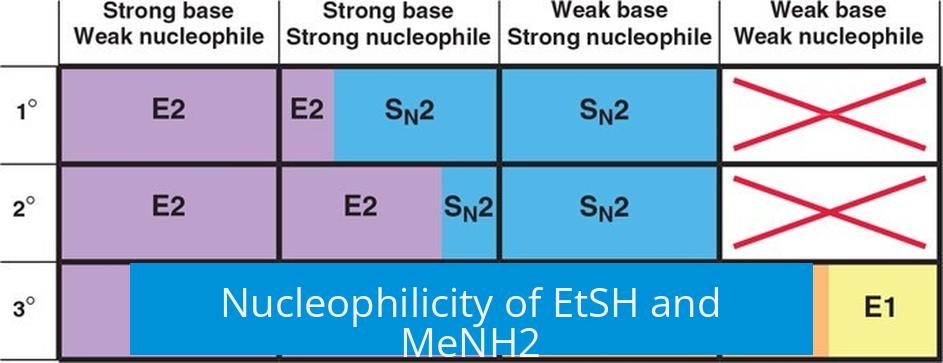
EtSH and MeNH2 are considered weak nucleophiles in their neutral forms. The mercaptan group (EtSH) normally acts as a reasonable nucleophile when deprotonated (EtS−), but in its protonated form, EtSH reacts more slowly. Similarly, MeNH2 (methylamine) is a neutral amine and exhibits moderate nucleophilicity, but its strength is limited compared to negatively charged species.
Effect of Protonation State
Protonation significantly influences nucleophilicity. For EtSH, the deprotonated thiolate ion (EtS−) is a much stronger nucleophile. The lack of deprotonation in EtSH reduces its reactivity in substitution reactions. MeNH2, as a neutral amine, can act as a nucleophile but is less reactive than anionic counterparts.
Preference for SN2 vs SN1/E1 Mechanisms
When reacting with alkyl halides, both EtSH and MeNH2 tend to favor SN1 mechanisms in substrates that can form stable carbocation intermediates. This is because the carbocation formed in SN1 provides a better target for these weaker nucleophiles. Specifically, EtSH may react more efficiently through the SN1 pathway due to its affinity for the positively charged intermediate.
In contrast, SN2 reactions require a strong nucleophile that can directly attack the electrophilic carbon. Given their weak nucleophilicity in neutral forms, EtSH and MeNH2 do not perform well in SN2 mechanisms, especially on sterically hindered substrates.
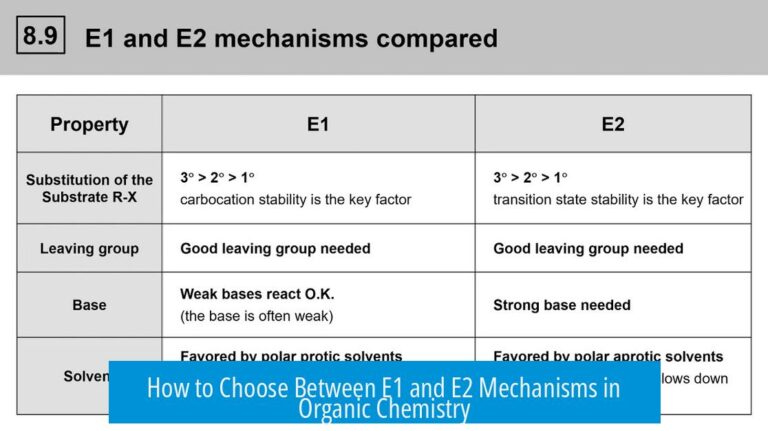
Elimination Reactions (E1/E2) Considerations
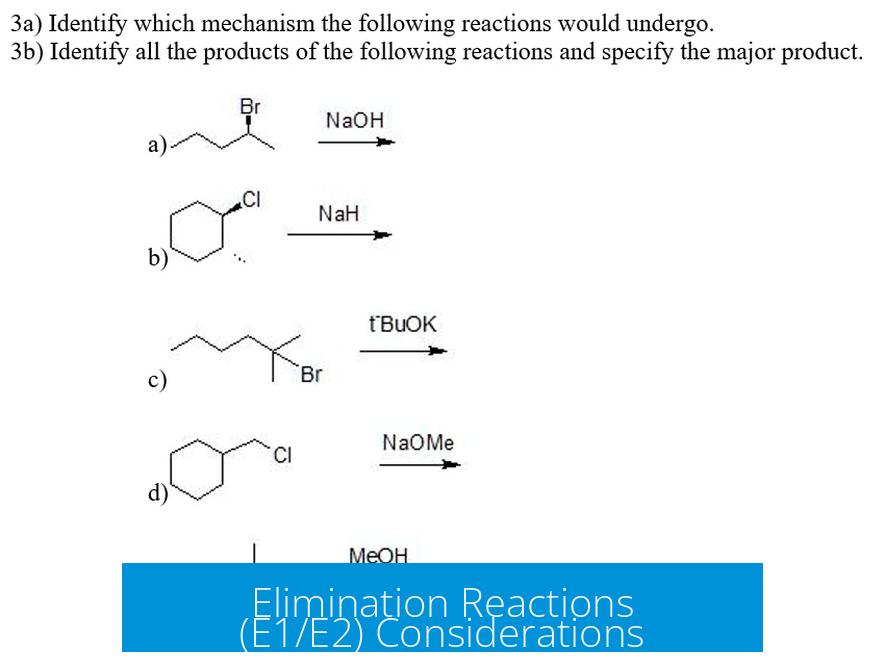
Elimination reactions require a strong base to abstract a proton, leading to alkene formation. Since both EtSH and MeNH2 are weak bases, elimination (E1/E2) is generally not favored under these conditions. The nucleophiles are more likely to engage in substitution than elimination.
Summary Table of Nucleophile Strength and Mechanism Preference
| Nucleophile | Strength (Neutral Form) | Preferred Mechanism | Elimination Favorability |
|---|---|---|---|
| EtSH (neutral) | Weak | SN1 (if carbocation forms) | Low |
| MeNH2 | Weak to Moderate | SN1 (carbocation intermediates) | Low |
| EtS− (deprotonated) | Strong | SN2 (favors backside attack) | Varies |
Key Points to Remember
- EtSH and MeNH2 are weak nucleophiles in their neutral forms.
- Deprotonation of EtSH to EtS− significantly increases nucleophilicity.
- SN1 is favored if a stable carbocation intermediate can form, benefiting these nucleophiles.
- SN2 is disfavored with weak nucleophiles and sterically hindered substrates.
- Elimination reactions require stronger bases and are not favored with EtSH or MeNH2.




Leave a Comment