Distinguishing Between Identical Molecules and Enantiomers
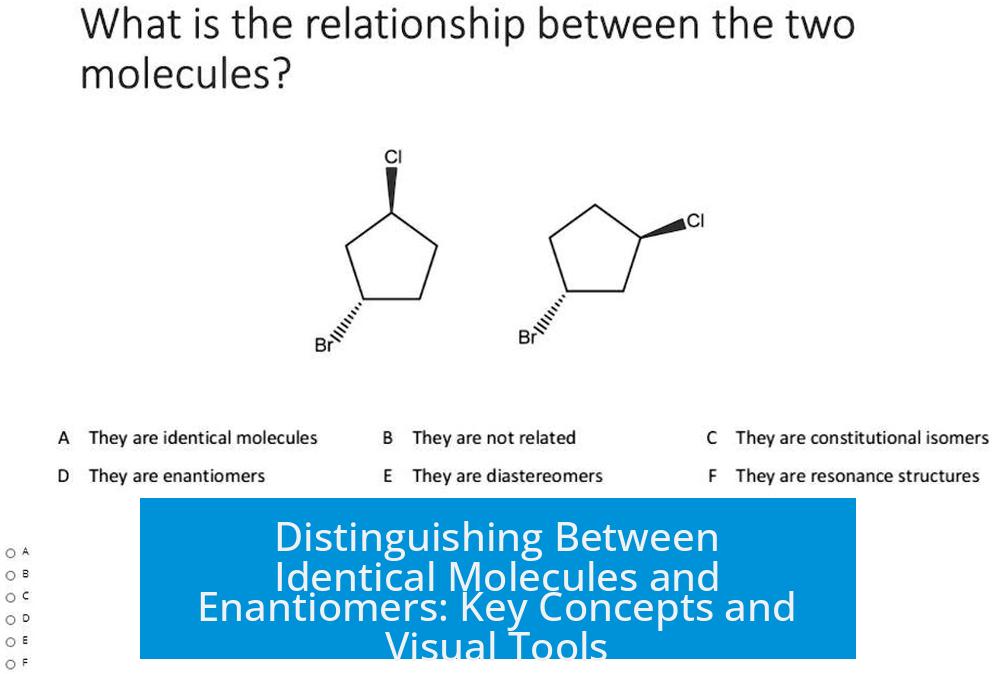
Two molecules cannot simultaneously be enantiomers and identical. Enantiomers are pairs of distinct molecules that are non-superimposable mirror images. Identical molecules, by contrast, are indistinguishable in both structure and configuration.
Defining the Relationship
Enantiomers represent a specific stereochemical relationship. They are analogous to siblings who share a family tie but are distinct individuals. This means that two molecules that are enantiomers have the same connectivity but differ in spatial arrangement, causing them to be mirror images that cannot be aligned perfectly.
Identical molecules, however, are effectively the same molecule in all respects. They have the same connectivity and three-dimensional arrangement. This means they can be superimposed perfectly on each other.
Visualizing Differences Using Molecular Flips
A useful method to distinguish identical molecules from enantiomers involves flipping one molecule like turning a page to observe changes in stereochemistry. For example, flipping can change the positions of groups from wedged (coming out) to dashed (going in) bonds.
- If flipping one molecule aligns all groups and bonds exactly with the other molecule, they are identical.
- If the flip creates a mirror image with opposite stereochemistry at chiral centers, the molecules are enantiomers.
Assigning Stereocenters (R/S Configuration)
When visualization is difficult, assigning absolute configurations (R or S) to each stereocenter provides clarity. Each stereocenter is described by its priority order of substituents, following Cahn-Ingold-Prelog rules.
If all stereocenters differ in configuration between two molecules (e.g., R in one corresponds to S in the other) and the molecules are mirror images, they are enantiomers. If all stereocenters match, the molecules are identical.
Mirror Relationship Analogy
Enantiomers relate to one another like left and right hands. When placed in front of a mirror, they appear similar, but one cannot be superimposed onto the other by rotation alone. This is the hallmark of enantiomerism in chiral molecules.
Key Takeaways
- Identical molecules are superimposable with matching stereochemistry.
- Enantiomers are non-superimposable mirror images with opposite stereochemistry at all chiral centers.
- Flipping molecular structures helps visualize differences in stereochemistry.
- Assigning R/S configurations confirms enantiomeric relationships when visualization fails.
- Enantiomers are distinct molecules, unlike identical molecules which are the same.
What is the main difference between identical molecules and enantiomers?
Identical molecules are the exact same compound. Enantiomers are two different molecules. They are related as mirror images but not the same.
How can flipping a molecule help distinguish enantiomers?
Flipping a molecule is like turning a paper over. It shows if the groups switch from dashed to wedged bonds or vice versa. This helps identify if two molecules are mirror images.
Why can’t one molecule be both identical and an enantiomer to itself?
Enantiomers are different molecules related like siblings. Just as you cannot be a brother to yourself, a molecule cannot be identical and an enantiomer at once.
What is the role of assigning S/R configurations in distinguishing molecules?
When flipping is hard to visualize, assigning S or R to each stereocenter helps. Different assignments mean molecules are enantiomers, while the same assignments mean they are identical.
How does the mirror analogy explain enantiomers?
Enantiomers relate like mirror images, just like your two hands. They look similar but cannot be superimposed on each other.




Leave a Comment