Understanding the NMR Spectrum of Acetylferrocene
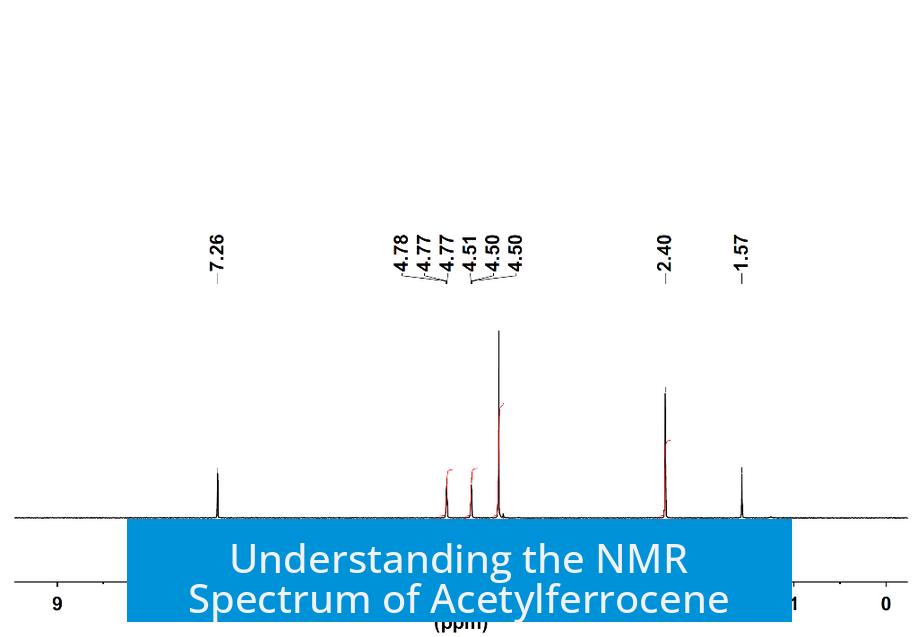
The 3H peak you labeled is the acetyl methyl (CH3) group, confirmed. The other three peaks correspond to the hydrogens on the two cyclopentadienyl rings: a 5H singlet for the unsubstituted ring and two 2H doublets from the substituted ring—so, no triplet appears here.
NMR Spectrum of Ferrocene: A Baseline
Ferrocene shows a simple pattern in NMR. It contains two cyclopentadienyl rings, each with 5 chemically equivalent hydrogens. Because all 10 hydrogens share the same environment, ferrocene produces a single, sharp peak integrating to 10 hydrogens.
Effect of Acetyl Substitution on the NMR Spectrum
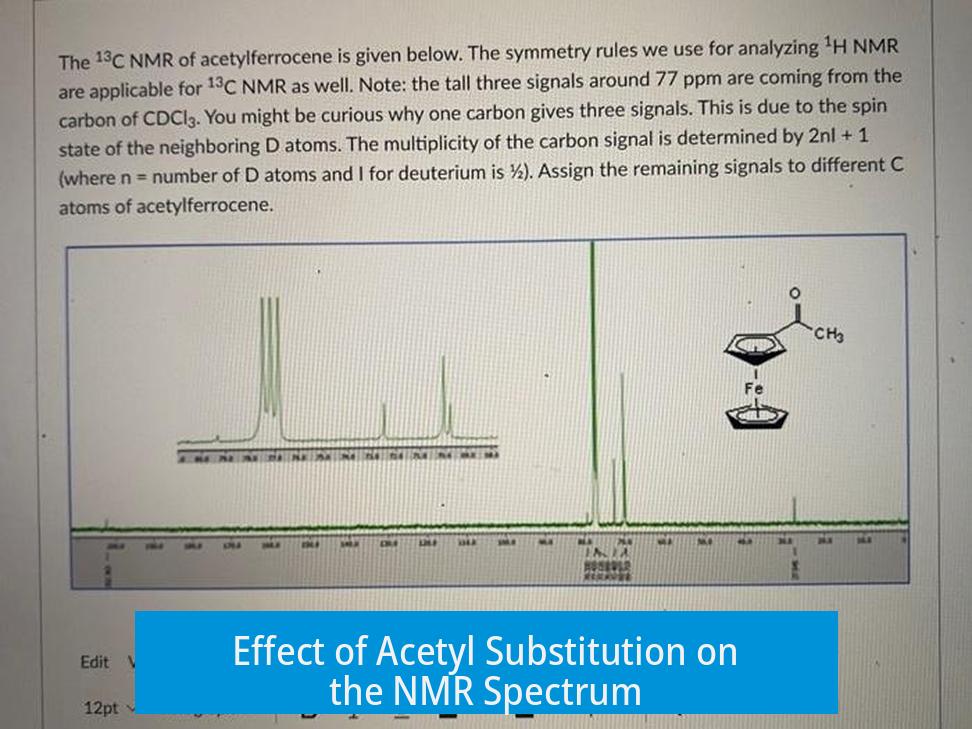
Attaching an acetyl group to one ring breaks the symmetry. This results in several distinct hydrogen environments:
- Unsubstituted Ring: All five hydrogens remain chemically equivalent, producing a large singlet integrating to 5H.
- Substituted Ring: The acetyl group causes the ring hydrogens to split into two sets of equivalent hydrogens: two closer to the carbonyl and two further away.
- Acetyl Methyl Group: The three hydrogens on the acetyl CH3 group appear as a distinct peak integrating to 3H.
Peak Types and Splitting
The substituted ring hydrogens are not equivalent to each other but form two groups of 2 hydrogens each. These produce two doublets, not a triplet or quartet. This splitting results from the coupling between neighboring hydrogens influenced by the acetyl group.
| Peak | Hydrogen Type | Integration | Splitting |
|---|---|---|---|
| Big singlet | Unsubstituted ring (5 hydrogens) | 5H | Singlet |
| Two smaller peaks | Substituted ring hydrogens | 2H each | Doublets |
| 3H peak | Acetyl methyl (CH3) | 3H | Singlet |
Acetic Acid Byproduct
Acetic acid formed during synthesis does not usually interfere with the NMR interpretation. Its signals appear separately and can be ignored in analyzing acetylferrocene.
Key Takeaways
- The 3H peak is the acetyl methyl group (CH3), a singlet.
- The unsubstituted ring hydrogens give one 5H singlet peak.
- The substituted ring hydrogens split into two sets of 2H each, showing as doublets.
- No triplet or quartet appears; hydrogens on the substituted ring couple to form doublets.
- Byproducts like acetic acid do not interfere with this pattern.
Why does acetylferrocene show multiple peaks instead of one single peak like ferrocene?
Adding an acetyl group breaks the equivalence of hydrogens on one ring. One ring remains intact with 5 equivalent hydrogens, producing a singlet. The other ring’s hydrogens split into two groups due to their different positions.
Is the 3H peak in the acetylferrocene NMR from the carbonyl hydrogens?
The 3H peak corresponds to the methyl group (CH3) of the acetyl. The carbonyl itself does not have hydrogens that show up in this region. The methyl hydrogens give a distinct 3H singlet peak.
Are the peaks from the substituted ring hydrogens triplets?
No, the substituted ring hydrogens appear as two doublets, each integrating for 2 hydrogens. They are not a triplet or a quartet because their environments differ due to proximity to the carbonyl.
What does the 5H singlet in acetylferrocene represent?
This peak is from the unsubstituted cyclopentadienyl ring. All five hydrogens are equivalent and appear as a single large singlet peak.
Why are there two doublets instead of one quadruplet for the substituted ring hydrogens?
The two hydrogens closer to the carbonyl group and the two hydrogens further away are chemically different. They couple differently causing two separate doublets rather than a single quartet.


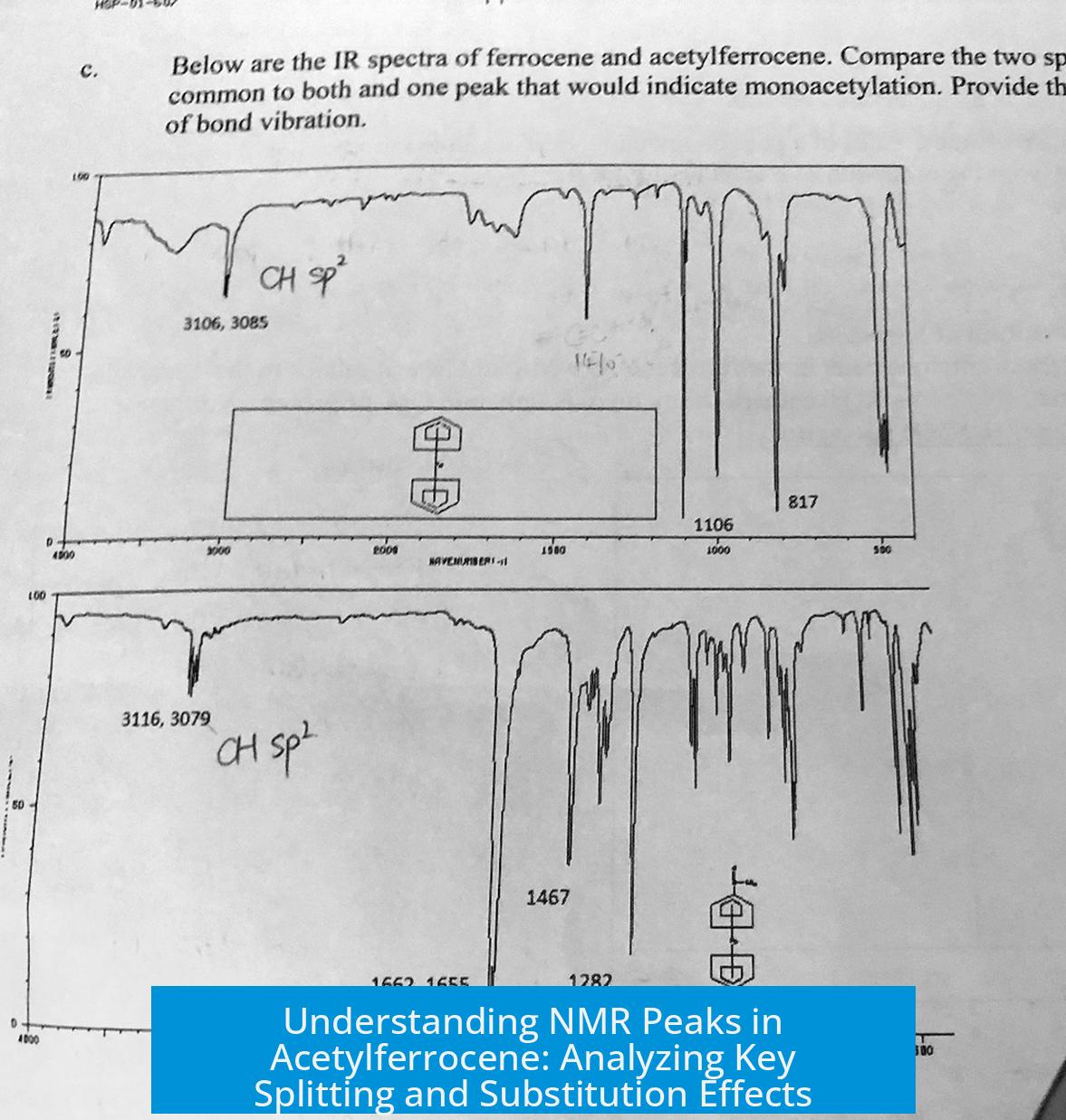

Leave a Comment