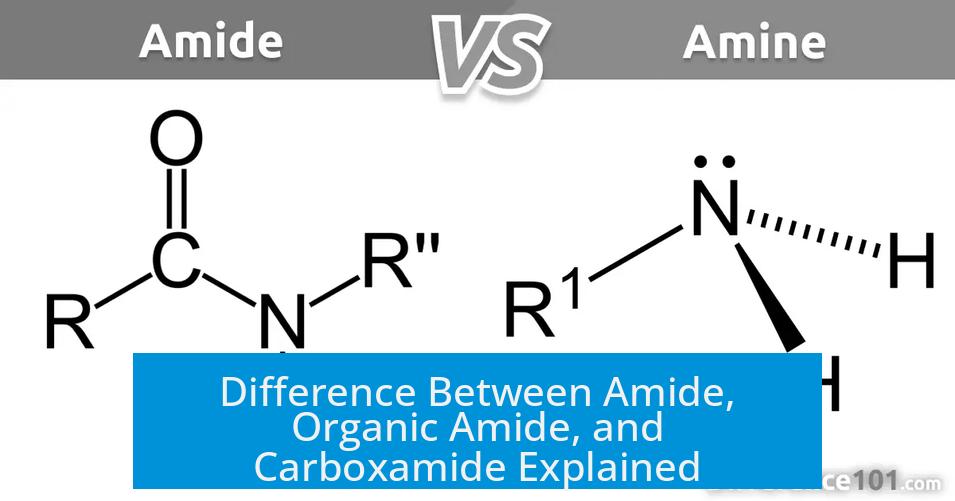
Difference Between Amide, Organic Amide, and Carboxamide
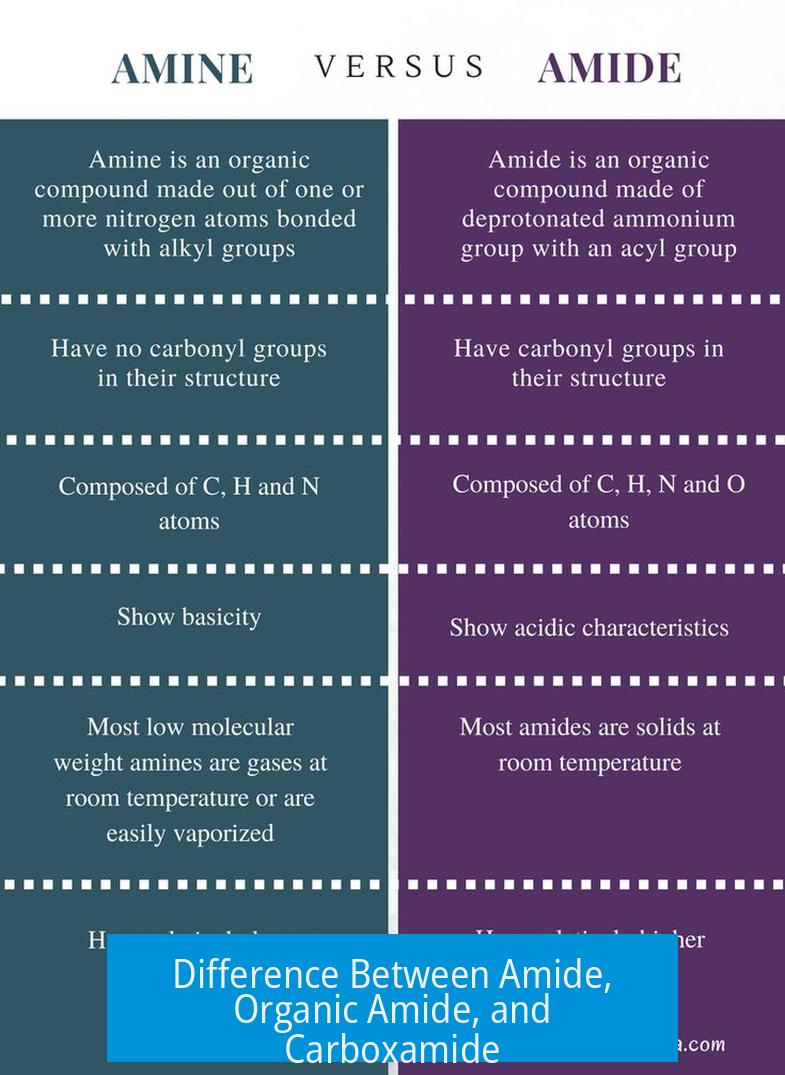
The term “amide” generally designates the carboxamide functional group but can also mean a nitrogen anion; “carboxamide” is the full name for this functional group, while “organic amide” is a rare, redundant term as all carboxamides are organic compounds.
1. Amide
In chemistry, “amide” primarily refers to a functional group containing a carbonyl (C=O) linked to a nitrogen atom (–CONH2). This group is a key feature of peptides and many organic molecules.
Additionally, “amide” can denote a nitrogen-centered anion (NH2−), found in reagents like lithium diisopropylamide (LDA). This dual usage depends on context.
Because anions usually end with “-ide,” such as chlorides and hydrides, the term “amide” in this sense aligns with typical chemical naming conventions.
2. Carboxamide
“Carboxamide” is the formal name for the amide functional group derived from carboxylic acids, essentially the same as “amide” in this context.
The longer term “carboxamide” helps avoid confusion with sulfonamides, distinct compounds that contain sulfur and nitrogen but have different properties.
3. Organic Amide
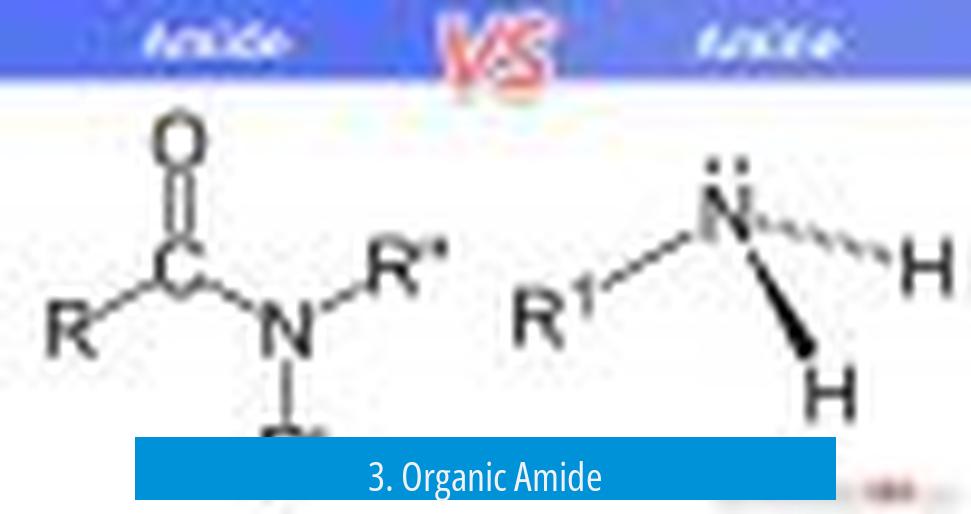
The term “organic amide” is uncommon and mostly redundant. Since carboxamides are organic by definition—organic compounds containing amide groups—the qualifier “organic” adds no new meaning.
Summary Table
| Term | Meaning | Common Usage | Clarification |
|---|---|---|---|
| Amide | Carboxamide functional group or nitrogen anion | Mostly functional group; anion usage in specific reagents | Generally means carboxamide; context matters |
| Carboxamide | Functional group containing –CONH2 | Clarifies functional group identity | Used to distinguish from sulfonamide |
| Organic amide | Rare term for amide in organic compounds | Not commonly used | Redundant because carboxamides are organic |
Key Takeaways
- “Amide” primarily means the carboxamide functional group but can also refer to a nitrogen anion.
- “Carboxamide” is the full, formal name for the amide functional group, used to avoid confusion.
- “Organic amide” is an uncommon, redundant term since carboxamides are inherently organic.
- Understanding usage depends on chemical context and structural distinctions.
The Difference Between Amide, Organic Amide, and Carboxamide: Clearing the Chemical Fog
Ever stumbled upon the words amide, organic amide, and carboxamide and wondered if they’re just fancy ways of saying the same thing? You’re not alone. These terms often swirl around in chemistry conversations like a thick mist, causing confusion. But here’s the truth wrapped up in a neat little package: Amide is generally the catch-all term for the carboxamide functional group, organic amide is an uncommon and mostly redundant phrase, and carboxamide is the full, explicit name for that functional group. Let’s unpack this with some clarity, a dash of fun, and practical insights.
What Is an Amide Anyway?
First, imagine chemistry as a language filled with shortcuts. The term amide is one of those shortcuts. In organic chemistry, an amide refers primarily to a functional group composed of a carbonyl group (C=O) bonded to a nitrogen atom (NR′R′′). The general formula looks like R−C(=O)−NR′R′′. Here, the Rs stand in for various groups—usually organic groups or hydrogens.
But hold on, amide doesn’t just wear one hat. It can also mean a nitrogen anion, like lithium diisopropylamide (LDA), often used in laboratories. That “-ide” ending typically means an anion, similar to chloride or hydroxide. So technically, “amide” can refer to two different chemical entities, but in most discussions, it points to the functional group found in many organic compounds.
Carboxamide: The Formal Name You Should Know
Now, if amide is the casual nickname, carboxamide is the full, formal name. It’s the amide derived specifically from a carboxylic acid. Why does this matter? Because there are other amide-like groups, such as sulfonamides, which involve sulfur atoms. Saying “carboxamide” helps highlight the carbonyl-nitrogen connection, distinguishing it from its sulfur-containing cousins.
For example, acetamide (CH3CONH2) is a simple carboxamide derived from acetic acid. In IUPAC naming, one usually adds “amide” to the parent acid name. So yes, carboxamide and amide frequently point to the same family of compounds. The longer name just provides precision.
So, What’s This “Organic Amide”?
Here’s where the confusion thickens. The term organic amide is surprisingly uncommon. Why? Because all carboxamides are inherently organic compounds. They contain carbon-based groups bonded to the amide functional group. So, calling something an “organic amide” is like saying “round circle”—redundant and unnecessary.
In other words, if you see the phrase “organic amide,” just know it usually means a regular carboxamide. Nothing extra special or different.
Getting Into the Structure and Chemistry of Amides
Want some cool chemistry trivia? The core amide structure—the C−C(O)−NR2 part—is perfectly planar. That means all atoms lie in one flat plane, making their bonding stable and unique. This flatness is due to resonance between two structures: a neutral form and a zwitterionic form where electrons shift around.
This resonance explains why amides are surprisingly unreactive compared to related compounds. They’re also weak bases, weaker than amines but stronger than carboxylic acids or aldehydes.
How Amides Behave in Real Life
Amides don’t just sit quietly in petri dishes. They’re crucial to everyday life and materials science. Their ability to form hydrogen bonds (both accepting and donating) makes them soluble in water, especially if they’re primary or secondary amides with N–H bonds.
On the practical front, amides form the backbone of proteins and materials like nylon, Kevlar, and other polyamides. Their peptide bonds, a type of amide bond, stitch amino acids into the proteins that build your muscles, enzymes, and yes, even your brain’s chemical machinery.
How Are Amides Made?
Their synthesis is pretty straightforward: chemists usually couple a carboxylic acid with an amine. Industrially, fatty amides are often produced by hydrolyzing nitriles. Knowing this is handy if you ever want to impress at a chemistry gathering—or just understand your shampoo ingredients better.
Summary Table: Amide, Organic Amide, and Carboxamide
| Term | Meaning | Usage | Common Confusions |
|---|---|---|---|
| Amide | Functional group (R−C=O−NR’) or nitrogen anion | General term in organic chemistry; mostly for carboxamide | Sometimes confuses with nitrogen anion amide or sulfonamide |
| Carboxamide | Full, precise name for the amide derived from carboxylic acids | Used to distinguish from sulfonamides and for formal naming | Often shortened to amide, causing overlap |
| Organic Amide | Uncommon and redundant term; all carboxamides are organic | Rarely used; mostly appears in informal or unclear contexts | May confuse beginners who think it’s a special class |
Why Does This Matter?
Understanding this terminology is more than just chemical semantics. If you’re a student, a chemist, or just a curious mind, knowing these differences helps you grasp literature clearly. Imagine reading a research paper talking about “amide hydrolysis” and wondering if it applies to your carboxamides or some exotic molecule. Spoiler: most times, it’s your everyday carboxamide.
Moreover, when discussing pharmaceuticals, polymers, or lab reagents, using precise terms reduces mix-ups. For example, sulfonamides are a class of antibiotics and are totally different from carboxamides, despite the similar sounding names.
Final Thoughts: The Chemistry Chat You Need
So next time someone drops “organic amide” casually, smile knowingly. You’ve got the skinny:
- Amide is mostly your carboxamide functional group but can mean a nitrogen anion.
- Carboxamide is the full name, handy when you want precision.
- Organic amide? Usually just a fancy, unnecessary way to say carboxamide.
Does this clear the air or raise more questions? Chemistry loves its little puzzles, but understanding the language helps you solve them faster.
Want to dive deeper? Explore how these amide groups participate in creating proteins or why their resonance makes them special in organic synthesis. Chemistry is a beautiful puzzle with pieces that connect in surprising ways.
What is the main difference between an amide and a carboxamide?
Amide is a shortened term for the carboxamide functional group. Both refer to the same structure, but “carboxamide” is used to avoid confusion with other amides like sulfonamides.
Does the term “amide” refer to anything besides the carboxamide group?
Yes. Besides the carboxamide group, amide can also refer to a nitrogen anion, like in lithium diisopropylamide (LDA). The suffix “ide” indicates this anionic form.
Why is the term “organic amide” rarely used?
“Organic amide” is redundant because all carboxamides are organic compounds by definition. The term is uncommon and not generally needed.
When should one use “carboxamide” instead of “amide”?
Use “carboxamide” to clearly distinguish the group from sulfonamides. It removes ambiguity when different amide types could be confused.



Leave a Comment