Diels-Alder Diene and Dienophile Relative Stereochemistry
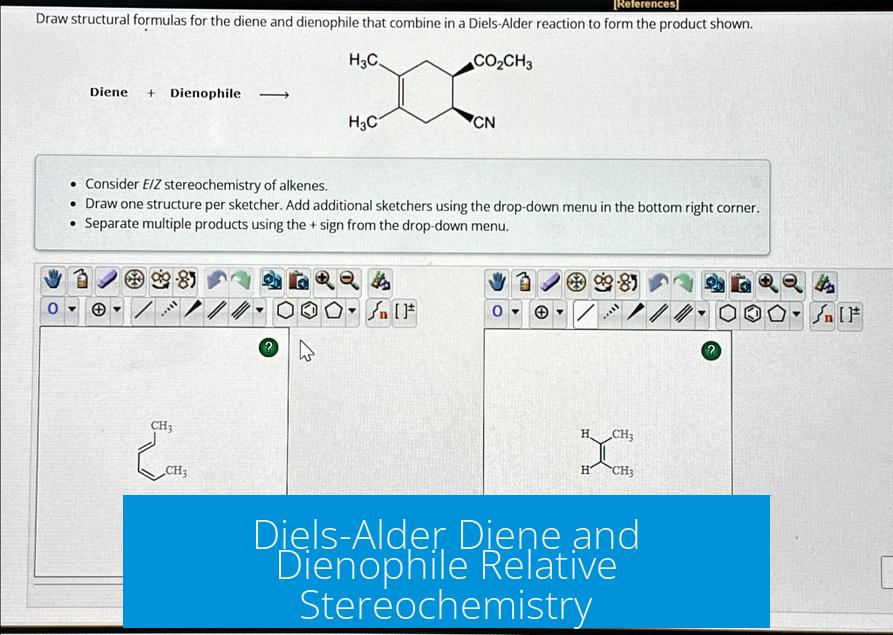
The relative stereochemistry in the Diels-Alder reaction depends on the planar nature of both the diene and dienophile and the way they approach each other, leading to the formation of stereoisomers such as endo and exo products.
Planarity and Molecular Approach
The Diels-Alder reaction involves a [4+2] cycloaddition between a diene and a dienophile. Both reactants have sp2 hybridized carbons making them planar molecules.
- The diene and dienophile exist as flat planes.
- During the reaction, the diene approaches the dienophile from either above or below its plane.
- This approach generates two possible stereochemical outcomes: enantiomeric products.
The planar geometry forces the reacting orbitals to align properly, controlling regiochemistry and stereochemistry simultaneously.
Endo vs Exo Addition: Definitions and Relative Positioning
Upon the diene’s approach, substituents attached to the dienophile influence the stereochemical outcome. The terms “endo” and “exo” describe this relative spatial relationship.
| Type of Addition | Substituent Orientation | Description |
|---|---|---|
| Endo | Substituent points toward the dienophile | The diene approaches so that substituents align underneath or near the π system of the dienophile. It often leads to the substituent being syn to the new ring system. |
| Exo | Substituent points away from the dienophile | The diene aligns such that substituents extend away from the dienophile, opposite to the π system. This often leads to anti stereochemistry relative to the ring. |
Both approach possibilities (from above or below) can produce these orientations, but the endo approach is typically favored due to secondary orbital interactions.
Transition State Alignment and Stereochemical Consequences
Analysis of the transition state shows that inside or endo substituents on the diene and dienophile align in a stacked fashion. This orbital overlap stabilizes the endo transition state.
- Inside (endo) substituents stack near each other.
- Outside (exo) substituents align on the opposite face.
- Bond formation pushes endo groups to the same side of the product, resulting in cis stereochemistry.
For example, in an endo addition, the “outside” group on the diene will have a cis relationship with the group on the dienophile. This arrangement results from the spatial constraints in the transition state.
Using Cyclic Dienes for Visualization
Cyclic dienes such as cyclopentadiene or furan make the stereochemical outcome easier to visualize and analyze.
- The diene terminals are locked together, imposing fixed relative positions.
- This rigidity simplifies understanding the endo/exo relationships.
- The stereochemical constraints highlight how substituents on the ring relate to those on the dienophile.
With their constrained geometry, cyclic dienes demonstrate how the endo product results from the substituent folding under the newly formed ring structure.
Effect of Substituent Size and Sterics on Endo/Exo Selectivity
Substituent bulk influences the preferred pathway in the Diels-Alder reaction.
- Larger groups may avoid the crowded environment under the dienophile, favoring exo products.
- Smaller or π-systems stabilize the endo transition state through secondary orbital interactions.
- Hence, sterics and electronics together modulate the stereochemical outcome.
Careful consideration of substituent size can predict whether the reaction will favor endo or exo addition products.
3-D Perspective and Stereochemical Clarifications
The planar structures and the three-dimensional movements of substituents explain the relative orientations observed.
- The direction of the diene’s attack plane determines which stereoisomer forms.
- Substituents move spatially to minimize steric clash or maximize interaction.
- Assigning endo or exo requires accurate 3-D models or molecular kits to visualize substituent positions.
Incorrect figures or misinterpretations often arise if the planar orientation of the diene and dienophile is misunderstood.
Key Points about Diels-Alder Diene and Dienophile Relative Stereochemistry
- The diene and dienophile are planar molecules due to sp2 hybridization.
- The diene can approach the dienophile either from above or below, generating enantiomers.
- Endo products form when diene substituents face toward the dienophile substituent, leading to cis stereochemistry.
- Exo products result when diene substituents point away from the dienophile substituent.
- The transition state features stacking of inside (endo) groups and outside (exo) groups, promoting orbital interactions.
- Cyclic dienes clarify relative stereochemistry by constraining diene termini.
- Steric bulk of substituents affects the endo/exo ratio by favoring less hindered pathways.
- 3-D visualization is essential for accurate stereochemical assignment.
What determines whether the Diels-Alder reaction yields endo or exo products?
It depends on the orientation of the substituent on the diene when it approaches the dienophile. If the substituent points toward the dienophile, the product is endo. If it points away, the product is exo. This applies for approaches from above or below.
How does the planar nature of the diene and dienophile affect stereochemistry?
Both are planar due to sp² hybridization. The diene can approach the dienophile from either above or below its plane. This creates enantiomeric products by changing the relative position of substituents.
Why do outside groups on the diene show cis stereochemistry to the dienophile in endo additions?
In the transition state, inside (endo) groups and outside (exo) groups align. When bonds form, this alignment pushes the outside group of the diene and the dienophile substituent to the same side, giving a cis relationship.
How can cyclic dienes help in understanding relative stereochemistry in the reaction?
Cyclic dienes connect the ends of the diene, restricting flexibility. This makes it easier to see how substituents relate in 3-D space and how the stereochemical outcomes arise during the Diels-Alder reaction.
Does the size of substituents influence the endo/exo selectivity in the reaction?
Yes, bulkier groups tend to affect whether the diene prefers the endo or exo approach. Larger substituents can favor the exo product by avoiding steric clashes during bond formation.


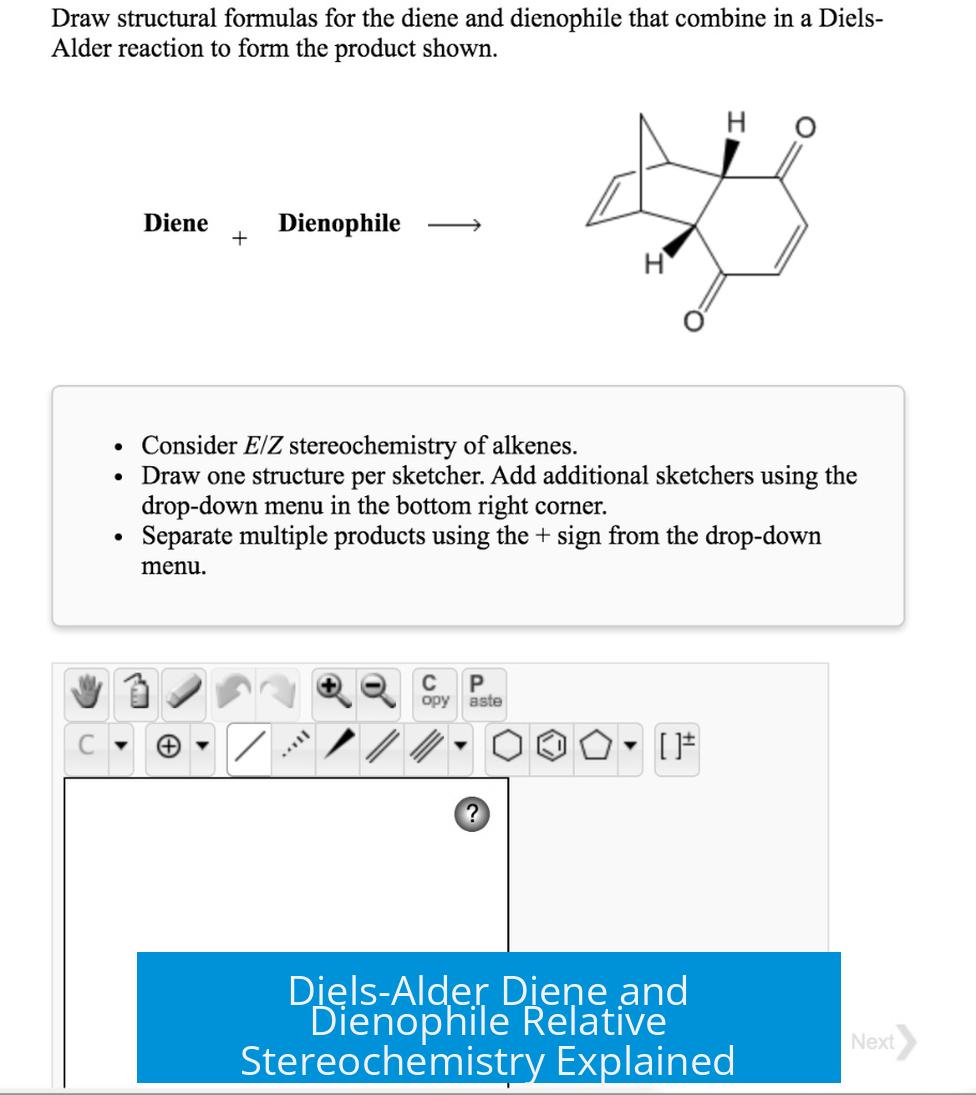


Leave a Comment