Determining Chirality in Cyclic Compounds
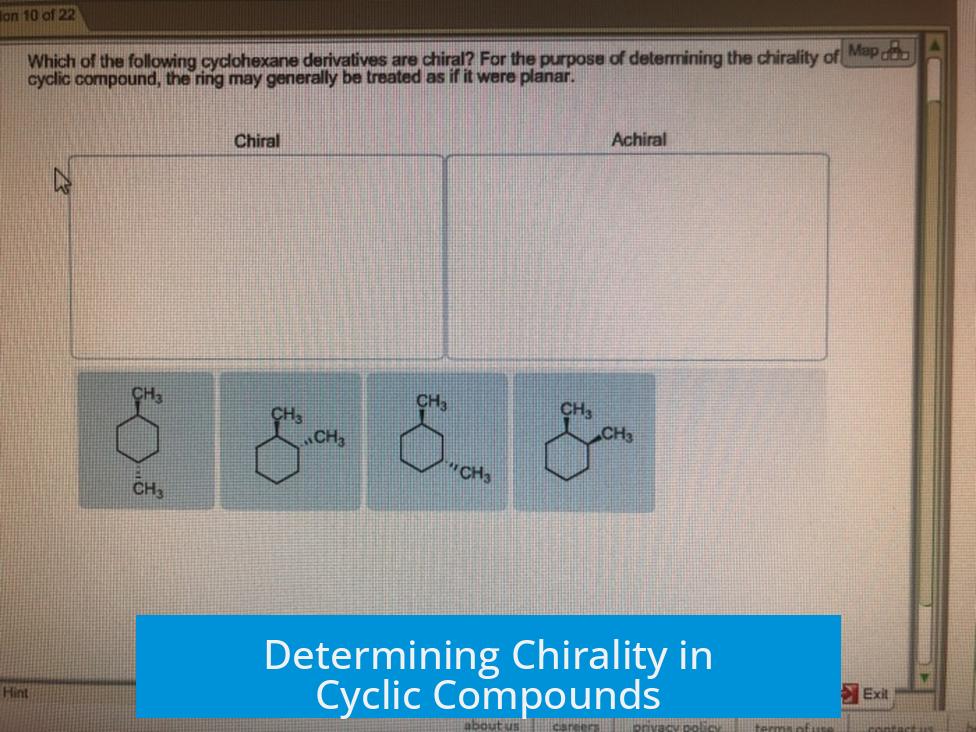
Is there a clear way to determine whether a cyclic compound is chiral? Specifically this one. Yes. Chirality can be assessed by examining the molecule’s symmetry elements and substituent arrangements. For cyclic compounds, identifying planes of symmetry, centers of inversion, or improper rotation axes is key.
General Rules for Chirality
- A molecule with a plane of symmetry or an inversion center cannot be chiral.
- If the molecule has an improper rotation axis (Sn), it is also achiral.
- The absence of improper rotation axes indicates the molecule is chiral.
- Chiral molecules may have proper rotational axes (Cn), such as C2 or C3.
Applying These to a Specific Cyclic Compound
When evaluating a cyclic compound, consider all possible stereochemical configurations using wedges and dashes to represent substituent orientations. If in all stereoisomers a plane of symmetry or improper rotation axis exists, the compound is achiral.
For the specific compound in question, this approach shows that all stereoisomers have a symmetry element. The molecule also exhibits two main diastereomers: cis (both substituents on the same side) and trans (substituents on opposite sides). However, neither form is chiral due to the presence of symmetry elements.
Symmetry and Chirality in Cyclic Systems
In cyclic compounds, symmetry often leads to achirality. Meso compounds exemplify this as they contain stereocenters but are achiral owing to internal symmetry. Conversely, if the molecule lacks any symmetry (no mirror planes, inversion centers, or improper rotation), it is likely chiral.
Conclusion for This Compound
This specific cyclic compound is achiral. Notably, the carbon atoms in the ring are not bonded to four different substituents, a necessity for chirality at stereocenters. The existing symmetry elements and identical substituent environments prevent chirality.
Key Takeaways
- Chirality in cyclic compounds depends on the absence of symmetry elements like mirror planes or improper axes.
- Analyzing all stereoisomers via wedge/dash notation helps reveal potential symmetry.
- Meso compounds illustrate achirality despite multiple stereocenters due to symmetry.
- Carbons must be attached to four different groups to be chiral centers.
- The specific cyclic compound discussed is achiral, confirmed by its symmetry and substituent patterns.




Leave a Comment