Understanding the sp Hybridization of Nitrogen in HCN
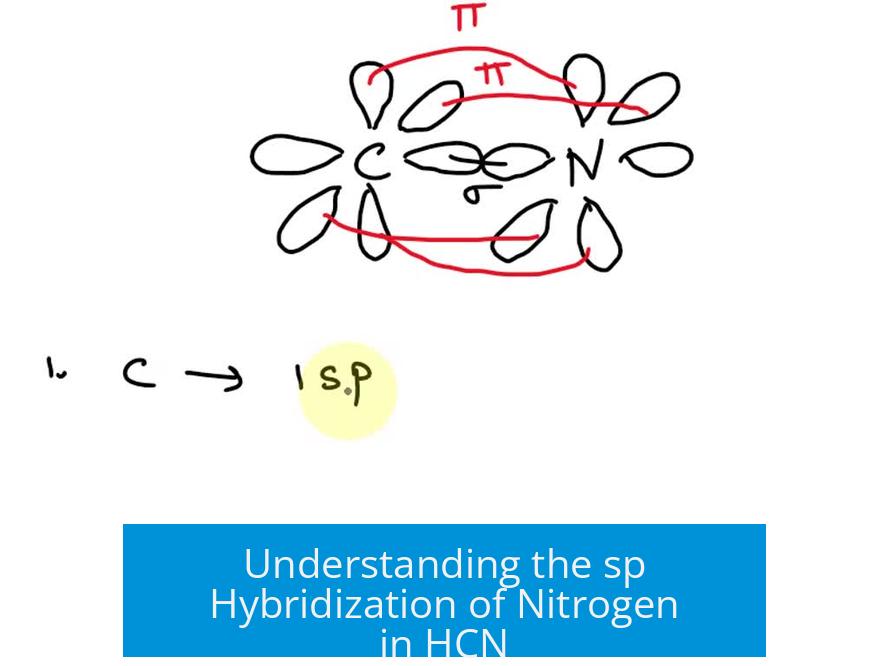
The nitrogen atom in hydrogen cyanide (HCN) undergoes sp hybridization due to having two regions of electron density: one from the triple bond with carbon and one from its lone pair, resulting in a linear molecular geometry.
Regions of Electron Density and Hybridization
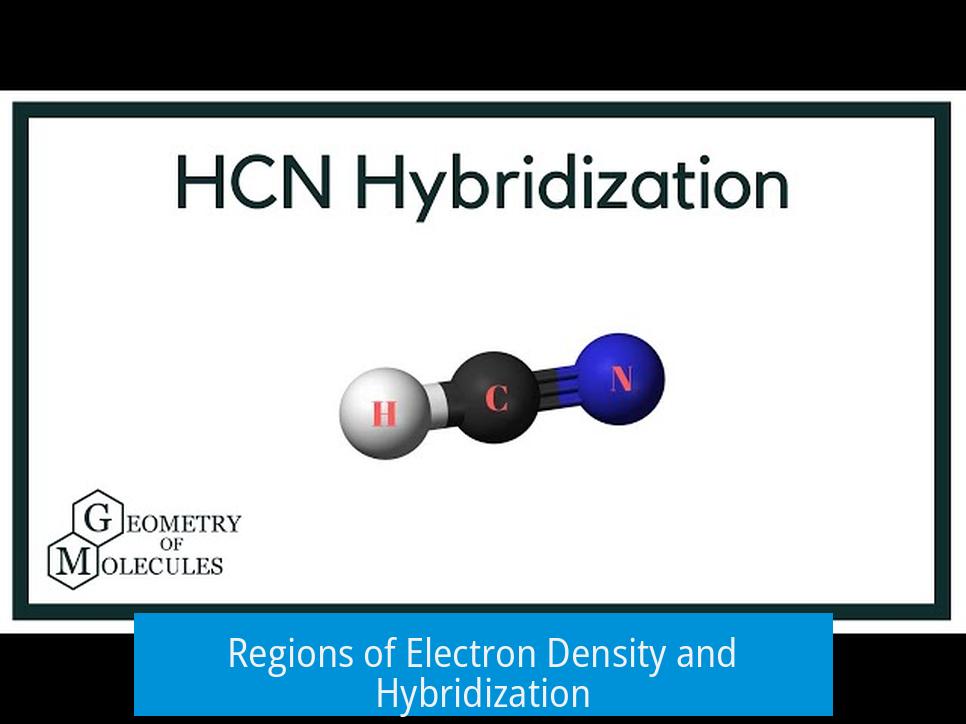
Hybridization arises from the number of electron density regions around an atom. These regions include bonds—single, double, or triple—and lone pairs.
- Each double or triple bond counts as one region of electron density.
- In HCN, nitrogen forms a triple bond with carbon, which counts as one region.
- Nitrogen also has a lone pair, forming a second region.
With two electron density regions present, nitrogen adopts sp hybridization. This involves the mixing of one s orbital and one p orbital to create two sp hybrids.
Geometry Resulting from sp Hybridization
The sp hybrid orbitals arrange themselves to minimize repulsion, creating a linear geometry with a bond angle of approximately 180°.
This geometry matches the observed structure of HCN, where nitrogen lies at one end, carbon in the middle, and hydrogen on the other end.
Lone Pair Placement on Nitrogen
In the sp hybridized nitrogen of HCN, the lone pair occupies one of the two sp hybrid orbitals.
The other sp hybrid forms the sigma (σ) bond with carbon.
The remaining unhybridized p orbitals on nitrogen participate in forming the π bonds of the carbon-nitrogen triple bond.
Visual Representation
A [diagram](https://slideplayer.com/slide/17084299/98/images/22/H+C+N+sp+Linear+2s+%2B+2px+Hydrogen+Cyanide%3A+Carbon+1s+2s+2px+2py+2pz.jpg) illustrating the sp hybridization in HCN aids in understanding orbital mixing and electron density distribution.
| Aspect | Detail |
|---|---|
| Number of Electron Regions on N | 2 (one triple bond + one lone pair) |
| Hybridization Type | sp |
| Geometry | Linear (≈180° bond angle) |
| Lone Pair Location | Occupies one sp hybrid orbital |
Key Points
- Nitrogen in HCN is sp hybridized due to two regions of electron density.
- Triple bond counts as one region; lone pair counts as the second.
- sp hybridization leads to a linear molecular shape.
- Lone pair resides in one of the nitrogen’s sp hybrid orbitals.
- Unhybridized p orbitals form the π components of the triple bond.



Leave a Comment