What Would This Molecule Be Named? C8H14

The molecular formula C8H14 represents several hydrocarbon isomers, including alkynes such as 2-octyne and 3-octyne, cyclic alkenes like cyclooctene, as well as bicyclic saturated compounds like bicyclo[2.2.2]octane. The exact name depends on the molecular structure, notably the presence and position of multiple bonds or ring systems.
Understanding the Formula C8H14
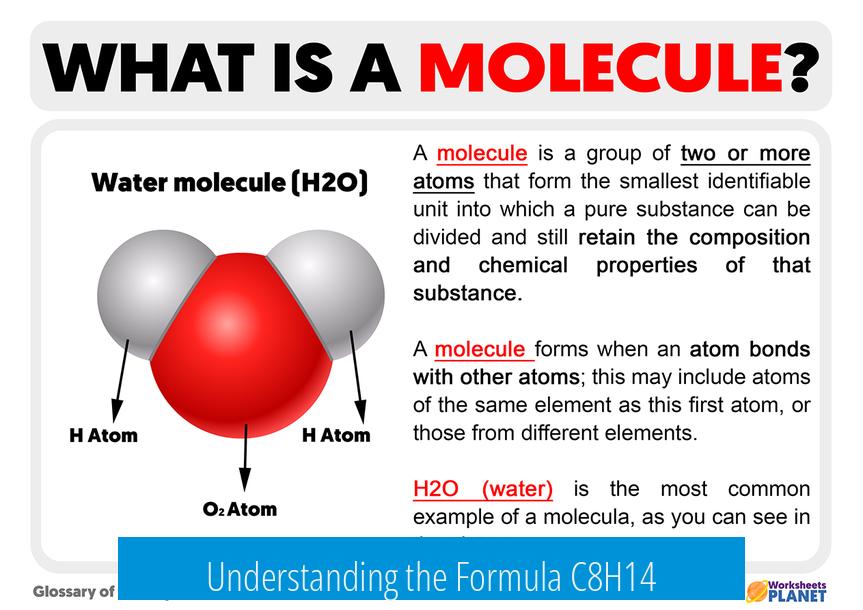
Certain hydrocarbons share the formula C8H14. This formula implies the molecule either has multiple bonds (double or triple bonds) or contains rings that reduce hydrogen count compared to saturated alkanes (like octane, C8H18).
- Maximum hydrogens for C8H molecules (alkanes): C8H18
- Each double bond or ring reduces hydrogens by two
The presence of only 14 hydrogens indicates three degrees of unsaturation: a combination of rings and/or multiple bonds.
Isomeric Possibilities for C8H14

1. Alkynes: 2-Octyne and 3-Octyne
2-Octyne and 3-Octyne are linear chain alkynes with a triple bond at the second or third carbon, respectively. These share the formula C8H14.
| Name | Structure Feature | Notes |
|---|---|---|
| 2-Octyne | Triple bond between C2-C3 | Also called methylpentylethyne or oct-2-yne; molar mass 110.20 g/mol |
| 3-Octyne | Triple bond between C3-C4 | Isomer of 2-octyne with similar properties |
These are typical alkynes. 2-Octyne is commonly referenced as a representative C8H14 molecule. It has density ~0.759 g/mL at 25 °C and a boiling point near 137 °C.
2. Cyclooctene
Cyclooctene is a cyclic alkene with eight carbon atoms arranged in a ring containing one double bond. It also fits the formula C8H14.
- Cyclooctene contains one ring and one double bond
- Its unsaturation count matches the molecular formula
- Its physical and chemical data are listed under CID 638079
Typically, cyclooctene is specified as cis- or trans- isomers due to restricted rotation at the double bond.
3. Bicyclic Hydrocarbons: Bicyclo[2.2.2]octane and Bicyclo[3.2.1]octane
Bicyclo[2.2.2]octane is a saturated bicyclic hydrocarbon with the formula C8H14. This name reflects three bridges connecting two bridgehead carbons, forming a cage-like structure.
| Compound | Type | Structural Notes |
|---|---|---|
| Bicyclo[2.2.2]octane | Saturated bicyclic | Compact, cage-like; analog to adamantane |
| Bicyclo[3.2.1]octane | Bicyclic | Another possible C8H14 isomer with different bridge sizes |
Due to the bicyclic structure, these compounds have hydrogens counted consistent with formula C8H14 despite being saturated.
Naming Accuracy and Common Confusions
Octane (C8H18) is sometimes mistakenly referenced for C8H14, but this is incorrect. Octane is fully saturated with no rings or double/triple bonds.
Cyclooctane (C8H16) and cyclooctene (C8H14) must not be confused. The suffix -tane indicates saturation; -ene indicates one double bond. C8H14 cannot be cyclooctane but fits cyclooctene perfectly.
Other names like “ethylhexane” or informal labels such as “houseane” or “cagane” liken complex bicyclic structures to shapes or cages but are not standardized IUPAC names.
Chemical Synthesis Note: Formation of 2-Octyne
2-Octyne is commonly produced by isomerization of 1-octyne, catalyzed by complexes such as Yb(II). This reaction shifts the triple bond position without altering molecular formula.
Summary of Key Points
- C8H14 corresponds to various hydrocarbons with three degrees of unsaturation.
- Common isomers include 2-octyne, 3-octyne (alkynes with a triple bond), cyclooctene (cyclic alkene), and bicyclic saturated compounds like bicyclo[2.2.2]octane.
- Octane (C8H18) is not a correct name for C8H14.
- Structures determine naming: triple bonds (alkyne suffix “-yne”), double bonds (alkene suffix “-ene”), and ring systems (cyclo- or bicyclo- prefixes).
- Bicyclo[2.2.2]octane represents a saturated, cage-like bicyclic structure fitting the formula exactly.
- 2-octyne and 3-octyne can be distinguished by the position of their triple bond along the carbon chain.
- Cyclooctene is the cyclic alkene variant having the same formula.
What Would This Molecule Be Named? Exploring the Identity of C8H14
So, what on Earth would the molecule with the formula C8H14 be called? Well, the straightforward answer points to bicyclo[2.2.2]octane, a bicyclic hydrocarbon featuring 8 carbons and 14 hydrogens arranged in a cage-like structure. But before you nod off, let’s dive into why this name fits and what other contenders are interesting to know about.
Imagine a molecule’s formula as a cryptic puzzle. C8H14 says it’s got 8 carbons and 14 hydrogens—noticeably fewer hydrogens than a fully saturated alkane with the same carbon count (C8H18)—hinting at rings or double bonds somewhere. That’s the opening clue.
Why Not Just Octane or Cyclooctane?
You might think C8H14 is something simple like octane or its cousin cyclooctane. But a quick reality check: octane is C8H18, fully saturated with hydrogen atoms stuffed onto a concrete 8-carbon chain. Cyclooctane has a ring but still boasts a formula of C8H16—two hydrogens more than our mysterious C8H14. That means C8H14 must have two hydrogens less than cyclooctane, suggesting the presence of unsaturation or extra rings, rather than a simple ring or straight chain.
Sometimes people toss around names like “cycloctane”—likely a casual slip of the tongue for cyclooctane. So, that’s a no-go for C8H14’s official title.
Could It Be Cyclooctene?
Another thought pops up: maybe it’s cyclooctene—a ring with a double bond. Cyclooctene’s formula matches C8H14 perfectly. However, considering various sources, the presence or absence of double bonds can shift the suffix from ‘-ene’ (double bond) to ‘-ane’ (single bonds only).
Interestingly, some suggest that the molecule is not cyclooctene because no double bonds seem apparent, leading to editing notes that “no tene” (no double bond) should imply an “-tane” suffix. That clashes with the C8H14 formula, though, which strangers to hydrocarbons hint denotes some unsaturation or bicyclic structure.
The Star of the Show: Bicyclo[2.2.2]octane
This is where the fireworks kick in. Bicyclo[2.2.2]octane steals the spotlight as the best fit for C8H14. What makes this molecule special? It’s a bicyclic hydrocarbon—think of it as two rings sharing some carbon atoms in a specific pattern. Its formula is perfectly in line with C8H14, explaining the hydrogen shortfall due to bicyclic constraints.
The numbers [2.2.2] describe how the carbons connect in those rings: three bridges connecting two bridgehead carbons, with lengths of two carbons each. This creates a compact, rigid cage, which resembles adamantane—a known sturdy hydrocarbon cage. The comparison to adamantane highlights the molecular stability and unique shape.
“Bicyclo[2.2.2]octaneClose to adamantane”
This cage-like arrangement reduces hydrogen count as the carbons bond more internally. It’s like designing a tiny molecular fortress. Chemistry buffs call this structural elegance “bicyclic,” and it’s a hallmark of compounds with formula patterns like C8H14.
Other Names and Possibilities to Ponder
- 1,4-Ethylhexane: Sometimes proposed as a derivative, this name hints at an alkane chain (hexane) with an ethyl side group. Yet, this name fails to clarify C8H14’s unsaturation or rings directly.
- 2-Octyne (Oct-2-yne): An alkyne with a triple bond at carbon 2. It matches C8H14 and is recognized by chemists for its density of 0.759 g/ml and boiling point near 137°C. It’s a linear molecule with a triple bond rather than a cyclic cage.
- 3-Octyne: Similar to 2-octyne but with the triple bond shifted, also fitting C8H14. A player in the alkyne family, it shares the same molecular formula but differs in structure.
Both 2-octyne and 3-octyne underline an important truth: molecular formula alone doesn’t give you the full picture. Positioning of bonds matters. Still, bicyclo[2.2.2]octane remains the prime bicyclic candidate, especially in discussions focused on strict ring structures that reduce hydrogen atom count.
Why Does Naming Matter Anyway?
You might wonder, “Why get bogged down naming these molecules like a chemistry detective?” The answer lies in communication precision. In labs, industry, and research, a clear name tells chemists about molecular structure, reactivity, and properties instantly. Calling C8H14 bicyclo[2.2.2]octane signals rigidity, bicyclic nature, and cage-like shape.
Meanwhile, “2-octyne” screams “alkyne with a triple bond,” suggesting different chemistry. Confusion can lead to errors in synthesis, safety, or application. Naming this molecule as bicyclo[2.2.2]octane isn’t quibbling; it’s about exactness.
How to Remember This Molecular Naming Puzzle
- Count your atoms. C8H14 indicates fewer hydrogens than a simple alkane or cycloalkane.
- Look for rings or multiple bonds. C8H14 usually involves bicyclic structures or unsaturations.
- Think “bicyclo” for rigid, cage-like, 2-ring bound carbon frameworks.
- Remember that minor name alterations (“cycloctane” vs. “cyclooctane”) can lead you astray!
Wrapping Up—Name That Molecule!
To recap, the molecule with formula C8H14 is most fittingly named bicyclo[2.2.2]octane. This name reflects its bicyclic cage structure with eight carbons and fourteen hydrogens. Other candidates like 2-octyne or cyclooctene exist but represent different structures with distinct properties.
Next time you see C8H14 scribbled in your notes, recall the tiny molecular fortress of bicyclo[2.2.2]octane. It’s chemistry’s way of telling us molecules can be elegant cages, not just straight or simple rings. Pretty neat, right?
Oh, and hey, if you’re in the lab struggling to name that mystery hydrocarbon, remember—sometimes it’s not just what atoms you have but how they’re cozying up that defines the superstar name.
What are some common names for molecules with the formula C8H14?
C8H14 corresponds to several isomers including 2-octyne, 3-octyne, cyclooctene, and bicyclo[3.2.1]octane. Each differs by structure and bonding.
Why is 2-octyne a typical name for a C8H14 molecule?
2-Octyne contains a triple bond at carbon 2, fitting the formula C8H14 as an alkyne. It’s a well-characterized example of this molecular formula.
Can cyclooctene be represented by the formula C8H14?
Yes, cyclooctene is a cycloalkene with one double bond in an eight-carbon ring, matching C8H14’s formula due to one ring and one double bond.
Is it correct to call C8H14 “octane”?
No. Octane has the formula C8H18. Labeling C8H14 as octane is incorrect since C8H14 is more unsaturated.
What is bicyclo[3.2.1]octane in relation to C8H14?
Bicyclo[3.2.1]octane is a bicyclic hydrocarbon with formula C8H14. It features two rings and matches the molecular formula precisely.





Leave a Comment