Carboxylic Acid Derivatives Practice

Carboxylic acid derivatives (CADs) share core reaction mechanisms, allowing focused study on their interconversions by understanding fundamental patterns. Mastering these patterns simplifies learning multiple transformations and their applications in organic synthesis.
1. Understanding Mechanisms of Carboxylic Acid Derivatives
The core of CAD reactions involves nucleophilic acyl substitution. Most conversions share a common sequence:
- Nucleophilic attack on the carbonyl carbon.
- Formation of a tetrahedral intermediate.
- Leaving group departure restoring the carbonyl.
By mastering this general mechanism, it becomes easier to predict outcomes for esters, anhydrides, acid chlorides, amides, and other derivatives. Recognizing that many transformations differ mainly in the leaving group’s nature reduces memorization.
2. Electrophilicity Order of Carboxylic Acid Derivatives
Understanding the electrophilicity order guides which CADs react faster and under what conditions. The common electrophilicity ranking is:
| More Electrophilic | Acid chlorides > Anhydrides > Esters > Amides | Less Electrophilic |
|---|
This trend relates to the leaving group ability and resonance stabilization. Acid chlorides have weaker resonance due to the electronegative chlorine, increasing electrophilicity. Amides are least reactive because the nitrogen lone pair donates electron density, stabilizing the carbonyl.
3. Reagents and Conditions for Conversions
Each CAD transforms into others by specific reagents and conditions.
- Acid chlorides to esters: React with alcohols under mild base or pyridine.
- Anhydrides to amides: Ammonia or amines in aqueous or alcoholic solutions.
- Esters to carboxylic acids: Acidic or basic hydrolysis.
- Amides to carboxylic acids: Strong acid or base under reflux.
These reactions can affect other sensitive groups. For example, acid chloride formation often uses thionyl chloride, which can react with alcohol or amine groups. Understanding reagent compatibility prevents side reactions.
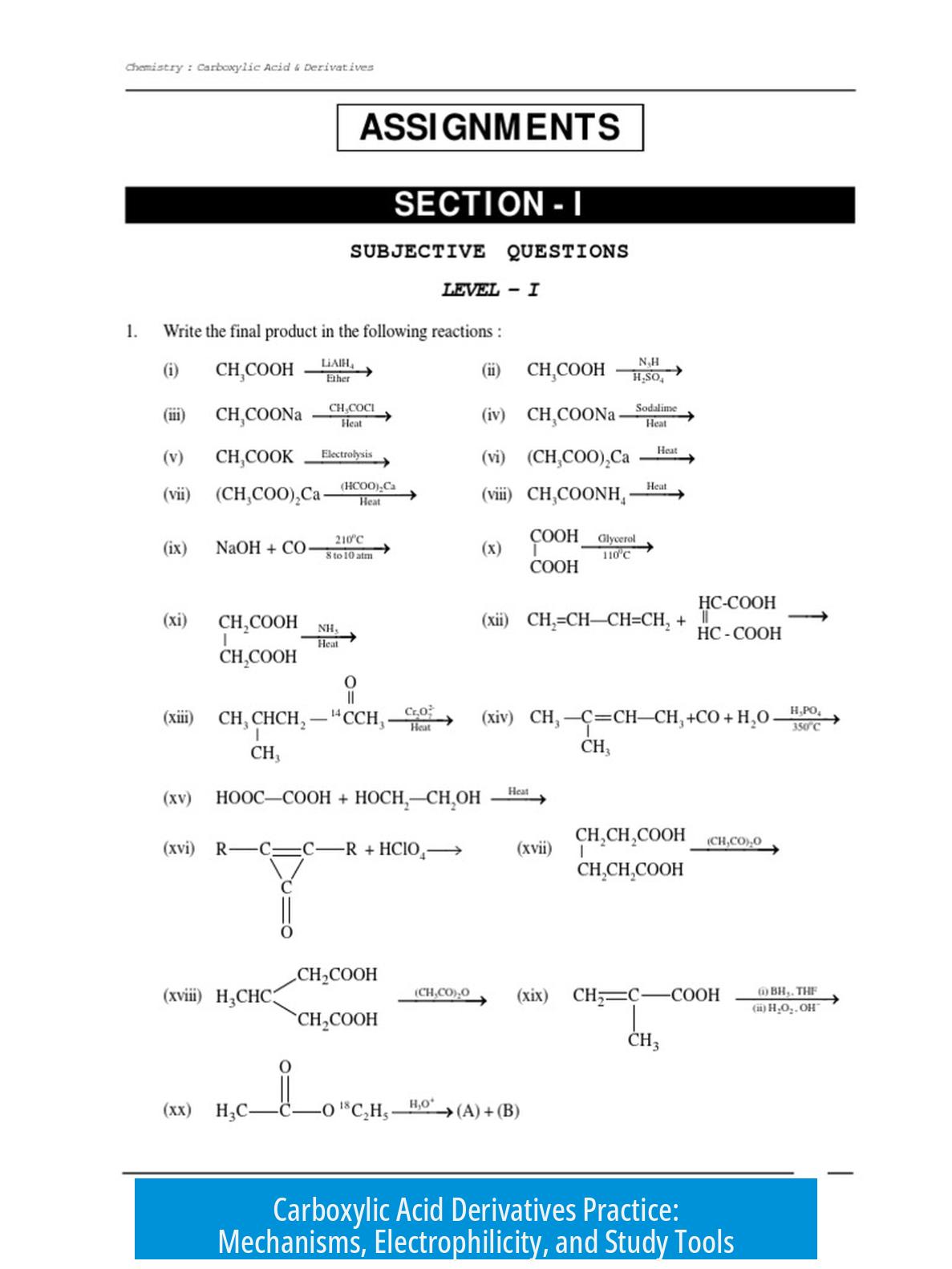
4. Study and Practice Tools
Use quizzes and apps dedicated to carboxylic acids to test knowledge. The Carboxylic Acids app offers practice problems to reinforce concepts.
Organize knowledge in a web or network format connecting mechanisms, reactivity, and reagents. Mapping these relationships aids retention and quick recall during problem-solving.
Additional problem sets focused on CADs help deepen understanding. Reviewing old assignments or textbooks with practice questions provides structured exercises.
Key Takeaways
- Most CAD reactions share a nucleophilic acyl substitution mechanism.
- Electrophilicity order: acid chlorides > anhydrides > esters > amides.
- Careful choice of reagents and conditions enables selective CAD interconversions.
- Use apps, quizzes, and concept maps to organize study and practice efficiently.




Leave a Comment