Assessing the Correctness of the Chichibabin Reaction Mechanism Drawing
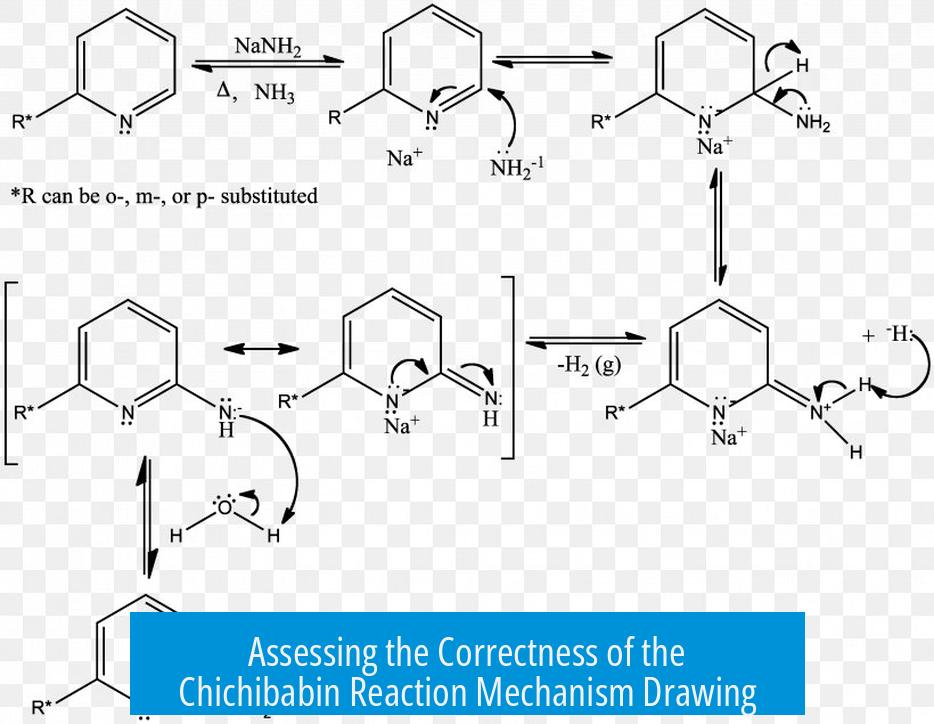
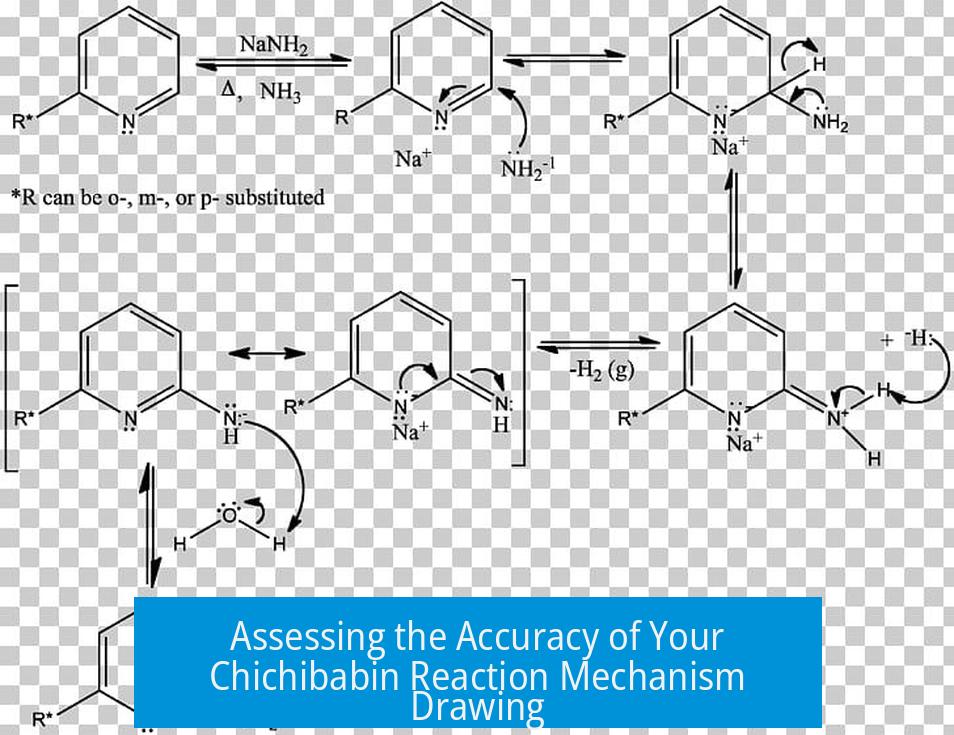
The mechanism you have drawn for the Chichibabin reaction appears mostly correct, but it contains several common errors in arrow pushing and electron movement depiction. The final protonation steps of the hydride are usually omitted because they do not represent steps in the core mechanism.
This review clarifies specific issues to help improve your mechanism depiction and enhance mechanistic accuracy.
Core Correctness and Final Steps
Your overall mechanism captures the key intermediates correctly. The displacement of a hydride ion from an aromatic ring and nucleophilic attack by an amide ion on pyridine or derivatives are central features that you likely included properly.
However, the last two steps drawn—protonation of the hydride ion—do not typically belong to the mechanistic core. Hydride leaving and proton capture occur quickly from trace protic sources and usually are omitted. Thus, omitting these steps aligns with standard practice and makes the mechanism cleaner.
Arrow Pushing: Common Errors and Corrections
- Arrow Origin and Target: Your initial arrows mistakenly show lone pairs attacking C-H bonds. Mechanistically, nucleophiles target hydrogen nuclei (protons), not the bonds themselves. For clarity and correctness, draw arrows from the nucleophile’s lone pair to the proton to represent proton abstraction.
- Resonance Structure Arrows: In resonance depictions, ensure arrows indicate proper electron movement. Arrows should start from a lone pair or bond and point to a bond or atom to denote bond formation or electron pair shifts. Your arrows appear reversed, moving from nuclei toward carbons, which misrepresents electron flow and hinders understanding.
- Electron vs Charge Movement: Mechanistic arrows represent electron movement, not charge movement. Avoid arrows implying charges move without electron pairs accompanying them. This clarifies that lone pairs relocate rather than the formal charges themselves.
Lone Pairs and Charges Representation
Lone pairs are crucial for illustrating electron movement. Omitting them can confuse readers about electron sources or sinks. Instead of just showing a negative charge symbol, explicitly draw lone pairs on atoms to indicate electrons’ positions.
For example, nitrogen’s conversion to an anion during the reaction involves gaining a lone pair. Including a curved arrow showing this formation supports proper mechanistic interpretation.
Avoid showing impossible intermediates such as a negative charge on a hydrogen atom without proper context, as hydride is a leaving group and not an attacking nucleophile on bonds.
Mechanistic Realism and Formalism
Keep in mind that arrow pushing serves as a formalism to represent complex orbital interactions in a simplified way. The actual reaction involves orbital symmetry and linear combination of atomic orbitals.
As long as your intermediates are correct, minor imperfections in arrow direction may not drastically affect the conceptual understanding. However, consistent and accurate arrow pushing enhances clarity and instructive value.
Remarks on the Reaction and Hydride Leaving Group
The Chichibabin reaction is unusual due to hydride acting as the leaving group. This behavior defies typical nucleophilic aromatic substitution behavior, which usually involves halide leaving groups. Recognizing this peculiarity helps explain why the hydride removal step is sometimes treated outside the core mechanism.
Hydride ions released in the mechanism rapidly protonate, but tracking this step in the drawing is not essential.
Summary of Key Improvements
| Issue | Correction |
|---|---|
| Incorrect arrow origin (lone pair attacking bonds) | Draw arrows from lone pairs to nuclei (protons) for proton abstraction |
| Arrows in resonance structures pointing incorrectly | Ensure arrows indicate electron pair shifts toward bond formation |
| Showing movement of charges without electrons | Show lone pairs moving, not just charges |
| Lone pairs omitted | Explicitly draw lone pairs where electrons move or reside |
| Hydride attacking bonds incorrectly | Show hydride leaving and free proton capture separately or omit |
| Missing arrow for nitrogen anion formation | Add arrows showing lone pair or charge buildup on nitrogen |
| Unnecessary hydride protonation steps | Exclude from mechanism for simplicity and accuracy |
Key Takeaways
- Start arrows from lone pairs targeting nuclei or bonds correctly, avoiding attack on C-H bonds directly.
- Use arrows properly in resonance to show electron pair shifts and bond formation.
- Depict lone pairs explicitly to represent electron movement, not just charges.
- Hydride as a leaving group is unusual; its protonation is often not part of the formal mechanism.
- Including missing arrows, especially for nitrogen anion formation, enhances mechanism accuracy.
- Arrow pushing is a formalism; focus on correct intermediates and electron flows for clarity.




Leave a Comment