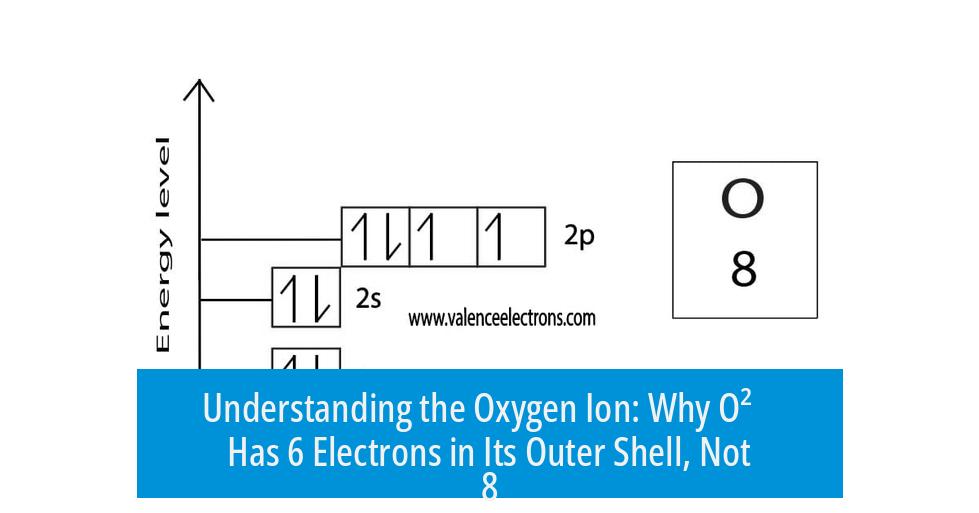
Understanding the Electron Configuration of the Oxygen Ion (O2−)

The oxygen ion O2− has 8 electrons in its outer shell, not 6, because it gains two electrons beyond the 6 valence electrons in neutral atomic oxygen, resulting in a full outer shell with the configuration 1s2 2s2 2p6.
Initial Electron Configuration of Neutral Oxygen
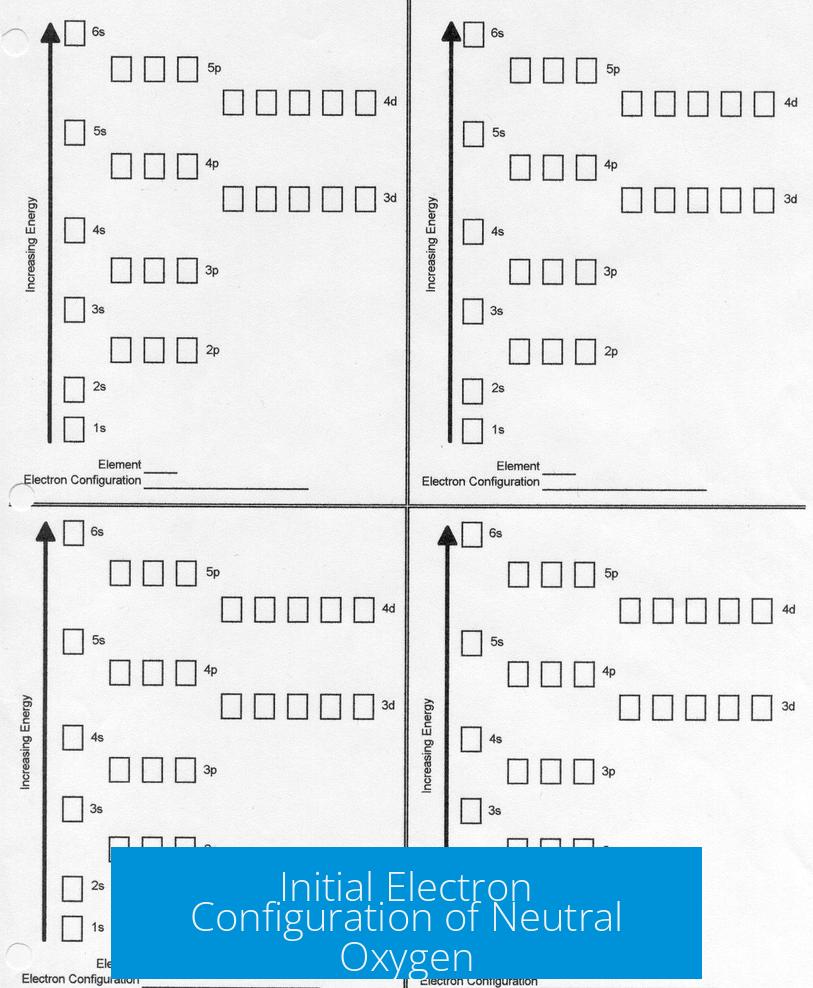
Atomic oxygen contains 8 electrons in total. These are arranged as 1s2 2s2 2p4.
- Two electrons fill the first shell (1s2).
- Six electrons occupy the second shell (outer shell): 2 in 2s and 4 in 2p.
Thus, neutral oxygen has 6 valence electrons. This arrangement is not fully stable because the outer shell can hold 8 electrons.
Formation of the O2− Ion
The oxygen ion O2− forms when oxygen gains two electrons from another atom or molecule.
- These extra electrons fill the vacant spaces in the 2p orbital.
- Now, the outer shell has 8 electrons: 2s2 2p6.
This results in the electron configuration 1s2 2s2 2p6, which is the same as neon, a noble gas.
Why Does the Ion Have a -2 Charge?
The -2 charge arises because oxygen now has 2 more electrons than protons:
| Particle | Count | Charge |
|---|---|---|
| Protons | 8 | +8 |
| Electrons | 10 (8 original + 2 gained) | -10 |
| Net charge | -2 |
The ion’s charge reflects extra electrons, not a change in proton number. The gain of 2 electrons gives oxygen a full and stable outer shell.
Common Misconceptions
- Thinking O2− has 6 valence electrons is incorrect. That count applies only to neutral oxygen.
- O2− gains electrons to complete its outer shell, achieving stable electronic structure.
- Its electron configuration changes to match that of a noble gas due to the two added electrons.
Examples in Ionic Compounds
- In magnesium oxide (MgO), the oxide ion exists as O2− paired with Mg2+.
- In sodium oxide (Na2O), two Na+ ions balance the charge of one O2− ion.
These compounds demonstrate how oxygen attains an octet by gaining electrons and forming ions.
Key Points
- Neutral oxygen has 6 valence electrons: 1s2 2s2 2p4.
- Oxygen ion (O2−) has 8 valence electrons: 1s2 2s2 2p6.
- The -2 charge shows oxygen gained two electrons, not that it originally had 8 electrons.
- Electron gain stabilizes oxygen by completing its outer shell.
- Ionic compounds like MgO contain the O2− ion with a full valence shell.
Why does neutral oxygen have 6 electrons in its outer shell?
Neutral oxygen has 8 electrons total. Two fill the first shell (1s²). The outer shell has 6 electrons (2s² 2p⁴). This is its natural, less stable state.
How does oxygen gain 2 electrons to form the O²⁻ ion?
Oxygen gains two extra electrons from another atom, filling its outer shell completely. This gives it 8 electrons in the outer shell, resulting in the O²⁻ ion.
Why is there confusion about 6 or 8 outer electrons in O²⁻?
It depends if you look at neutral oxygen or O²⁻ ion. Neutral oxygen has 6 outer electrons; O²⁻ has 8 outer electrons after gaining 2 electrons.
Does the -2 charge mean oxygen has extra protons?
No. The -2 charge means oxygen has gained 2 electrons without changing its number of protons. The proton count remains 8, but electrons increase to 10.
What is the electron configuration of O²⁻?
The O²⁻ ion has the configuration 1s² 2s² 2p⁶, showing a full outer shell with 8 electrons for stability.



Leave a Comment