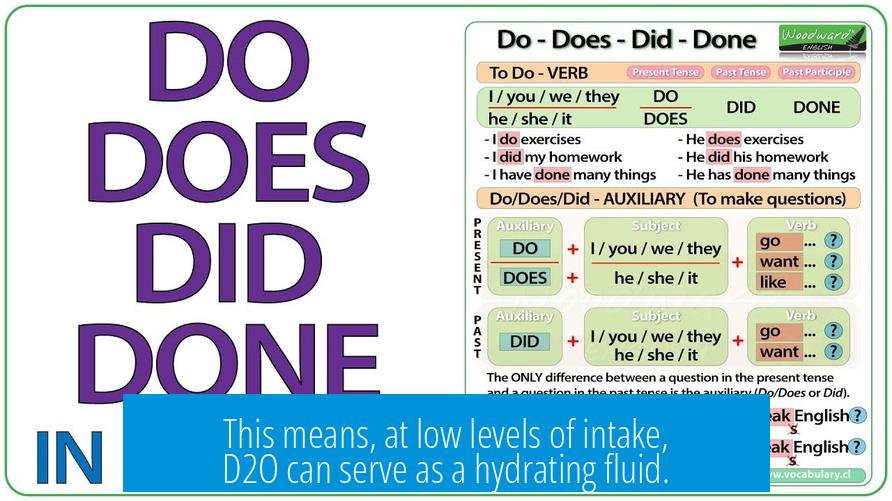
Does D2O “Hydrate” You?

Yes, D2O (deuterium oxide or heavy water) does hydrate you in the literal sense, as it can replace the water lost from the body through sweating and urination. However, it is not advisable to use D2O as a hydrating agent because it exhibits toxicity at concentrations above about 20%, disrupting normal biochemical and metabolic pathways essential for life.
Understanding D2O as a Hydrating Agent

Deuterium oxide, commonly called heavy water, differs from regular water (H2O) by substituting hydrogen atoms with deuterium, a heavier isotope of hydrogen. Physically, it behaves similarly to regular water, making it capable of hydrating the body by replenishing lost fluids.
- Consuming D2O can quench thirst just like regular water.
- D2O enters bodily fluids and replaces lost water from sweat, urine, and other excretions.
- The hydration effect from D2O is due to its physical similarity to H2O, thus fulfilling the body’s need for fluid balance.
This means, at low levels of intake, D2O can serve as a hydrating fluid.
Toxicity and Metabolic Impact of D2O
While D2O hydrates at small doses, its difference at the atomic level causes biochemical complications at higher concentrations. Research from animal studies and biochemical analyses reveals several key issues:

- Enzymatic Rate Changes: D2O affects enzyme kinetics by altering rate constants. Chemical bonds involving deuterium are stronger and harder to break, slowing essential biochemical reactions. This leads to disruption of metabolic pathways.
- Metabolic Disruption: Large amounts of D2O slow down metabolism. The organism’s cellular functions are interrupted, particularly cell division and DNA replication.
- Cellular Toxicity: Exposure beyond roughly 20% body water deuteration in lab animals leads to impairment of vital processes and eventually death.
- Non-Radioactive Toxicity: The toxicity arises not from radioactivity but from the isotope effect—the mass difference between hydrogen and deuterium affecting reaction dynamics.
In summary, D2O’s heavier atomic weight results in stronger chemical bonds, which impede reaction rates and create physiological stress when it replaces normal water in cells.
Animal Studies and Biological Observations

Experiments conducted in the 1940s provide insight into D2O’s biological effects. Key findings include:
- Rats tolerated up to about 20% of their body water replaced by D2O; higher percentages led to fatal metabolic disruptions.
- The Manhattan Project scientists attempted to use deuteration to protect organisms against radiation by replacing their body water with D2O. Bacteria could be deuterated; some algae survived with difficulty; frogs did not survive the transition.
- The strategy of deuteration as radiation protection proved impractical for complex organisms due to the toxicity and lethality of high D2O levels.
These studies underscore the limited tolerance of animals to D2O and illustrate that while low doses are manageable, large-scale replacement of body water is incompatible with survival.
Practical Advice on D2O Consumption
D2O is not recommended as a drinking water substitute for several practical reasons:
- Toxicity Risk: D2O is poisonous in high concentrations, potentially fatal if consumed excessively.
- Expense: Heavy water is much more costly to produce than regular water.
- Inconvenience: It does not provide health advantages over H2O and may disrupt normal biological functions.
Consequently, it is prudent to avoid exclusive or substantial consumption of D2O. Regular water remains the safe and effective choice for hydration.
Scientific Literature and Resources
For those interested in further exploration of D2O’s effects, the following resources provide detailed scientific information:
Summary: Key Points About D2O and Hydration
- D2O can hydrate the body by replenishing fluid lost through normal processes.
- Toxicity arises at about 20% replacement of body water with D2O due to slowed biochemical reactions and metabolic disruption.
- Animal studies reveal lethal effects of high D2O concentrations.
- The toxicity mechanism is related to isotope effects, not radioactivity.
- Consuming small amounts of D2O is theoretically hydrating but not recommended.
- Regular water (H2O) remains the best choice for safe and effective hydration.
Does D2O “Hydrate” You? The Heavy Water Hydration Conundrum
Yes, D2O can hydrate you—in the literal sense. Drinking D2O (heavy water) replaces lost body water just as regular H2O would. It’s as straightforward as that. If you sweat, pee, or cry out of frustration after a tough day, sipping on D2O would physically restore your fluid balance.
But let’s not rush to chug the stuff just yet. There’s a devilish twist in the tale, and it’s heavier than you might think—literally. Sip on, curious reader, and unravel the quirks of deuterated water hydration.
What Exactly Is D2O and How Does It “Hydrate”?
D2O, or heavy water, differs from normal water because the hydrogen atoms it contains are actually deuterium isotopes — hydrogen atoms with an extra neutron. This slight weight bump changes some physical and chemical properties. But in terms of hydration, it’s still water. The body can use it to replace fluids lost through sweat or urine.
Imagine you’re thirsty after a run. A swig of D2O will moisten your throat and refill your cells’ water supplies just fine. Your body’s cells don’t care much if the hydrogen is a bit heavier—they just need the H2O molecule for all the usual metabolic magic.
But Wait, There’s a Toxic Catch!
Here’s where it gets murky. While small doses of D2O won’t harm you, consuming too much is dangerous. Studies from the 1940s reveal rats tolerated only up to about 20% of their body water replaced with D2O. Beyond that, metabolic chaos ensues.
What’s going on inside? The chemical bonds involving deuterium are harder to break than those with regular hydrogen. This means enzymatic reactions take a hit—slowed, disrupted, or simply stuck. Since enzymes power virtually every biological process, this “isotope effect” can grind cellular metabolism to a halt. Cell division stalls. Important biochemical pathways jam up. Eventually, it’s lethal.
So, substituting 100% of your body’s water with D2O? Bad idea. But the small amounts we might accidentally ingest, say in labs or during experiments, won’t immediately turn you into a frog that “croaks” (pun intended). Speaking of frogs, alumni of the Manhattan Project had a go at deuterating living organisms to provide radiation resistance—bacteria and algae managed but frogs died rather unceremoniously.
Why Don’t We Just Drink D2O Exclusively to Stay Hydrated?
Aside from the toxicity risks, D2O is no bargain at the supermarket. It costs a pretty penny, making it impractical for everyday hydration. Also, its potential to disrupt metabolism makes it a less-than-ideal choice compared to regular water.
Think of it this way: would you rather spend your hard-earned cash on something that hydrates with a side of slow poison? Or stick to conventional water, the trusty liquidity that’s cheap and safe? The answer’s obvious.
A Quick Trip Down the Diet Soda Confusion Lane
Since we’re talking about hydration curiosities, here’s another question that often bubbles up: Does diet soda hydrate you?
Short answer: Yes, diet soda can hydrate you. It consists of about 99% water. Plus, despite the presence of caffeine (a mild diuretic) and artificial sweeteners, research shows moderate intake doesn’t significantly dehydrate you.
So that fizzy, calorie-free sip can count toward your daily fluid intake—though health experts suggest water is still your best friend. Why? Because carbonation and sweeteners might tempt you into drinking more, but they don’t add nutritional value, and some people find the fizz less quenching than plain water.
A good guideline: enjoy diet soda in moderation, listen to your body’s thirst signals, but prioritize plain water for optimal hydration effects.
How Does D2O Compare to Regular Water on a Biological Level?
| Feature | Regular Water (H2O) | Heavy Water (D2O) |
|---|---|---|
| Molecular Weight | ~18 g/mol | ~20 g/mol |
| Hydration Capability | Replaces body water effectively | Also replaces body water physically |
| Metabolic Effects | No disruption to enzymes | Disrupts enzymatic reactions at high concentration |
| Toxicity Threshold | None | About 20% body water replacement toxic |
| Price | Cheap and accessible | Expensive and specialized |
The Takeaway: Should You Let D2O Quench Your Thirst?
If you’re sitting in a science lab, contemplating a strange swap of your regular water for D2O, a cautious “no” is the polite, expert-backed answer.
Yes, it will hydrate in the straightforward sense: your cells get the water they need, thirst subsides, and you feel refreshed for a moment. Nevertheless, your enzymes and cells might silently groan as their typical chemistry slows down. Prolonged or excessive consumption? Big no-no.
Feeling adventurous? Consider this: the mere fact that D2O can hydra–er, dydrate you at all baffles many. After all, it’s water with a weighty twist that upends basic biological chemistry at higher doses. Think of it as hydration with a strong side of molecular mayhem.
Your Hydration Game Plan
- For daily hydration: Stick to clean, fresh H2O—the classic and proven hero in the story of your health.
- For the curious scientists and adrenaline junkies: Small, controlled exposure to D2O might be tolerable but certainly not advisable to replace your primary fluid source.
- Avoid large D2O intake: Above 20% body water replacement with D2O can disrupt metabolism and lead to serious health risks.
- Don’t fall for myths: Heavy water isn’t radioactive; its toxicity stems purely from the isotope’s effect on chemical reaction rates within your body.
- If you want fizz, diet soda gives hydration with a taste twist. It’s no replacement for water but can supplement fluid intake reasonably.
Hungry for More Science?
Dive into the 1970s research published in this article for a deep-dive on heavy water’s physiological effects. Additionally, Thunderfoot’s video offers a thorough and entertaining exploration of D2O’s quirks in biology and chemistry.
Final Thoughts
So, does D2O hydrate you? Yes, in a direct, physical sense it does hydrate. But it’s a wild card with a toxic tipping point. Drink it like regular water, and your cells will get liquid love, but your enzymes might just throw an isotopic tantrum.
From frogs that didn’t survive to rats that tolerated just a fraction, the scientific story leans towards “interesting but dangerous.” For everyday thirst, keeping one foot firmly in the domain of H2O remains the best—and safest—bet.
And remember, whether it’s water or soda, hydration is about balance. Listen to your body (not your chemistry teacher), and keep your liquids simple, good, and well… liquid.
Does D2O actually hydrate the body like regular water?
Yes, D2O can hydrate you by replacing lost water in your body. It works like regular water in hydrating because it fills the body’s water needs.
Is drinking D2O safe for hydration purposes?
Not really. Small amounts might be safe, but consuming large amounts (around 20% or more) disrupts metabolism and is toxic.
Why does D2O become toxic at higher levels?
The heavy form of water slows or stops key biochemical reactions because chemical bonds with deuterium break down slower. This disrupts cell functions and division.
Have experiments shown how animals react to D2O consumption?
Yes. Rats tolerate only up to 20% D2O. Other animals like frogs could not survive high D2O exposure. Toxic effects limit its safe use.
Should I ever use D2O instead of regular water for hydration?
No. It’s expensive and harmful in large amounts. Regular water is safer and better for hydration.





Leave a Comment