Is HClO3 a Weak Acid or Strong Acid?
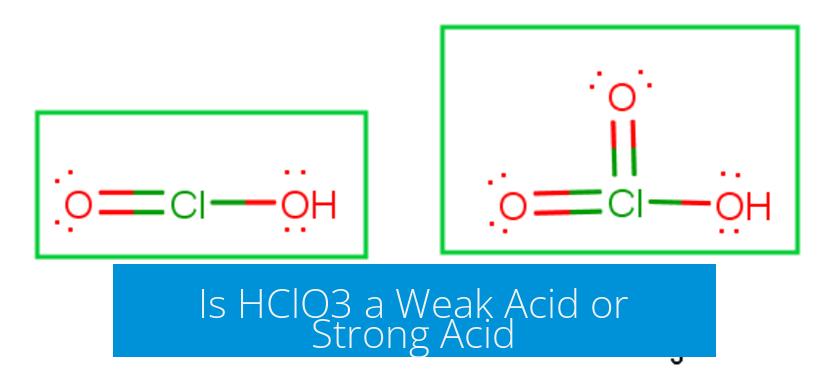
HClO3 is classified as a strong acid. It has a pKa value around -1, indicating it dissociates almost completely in water. This means it readily donates protons (H+) when dissolved, a key trait of strong acids.
Understanding Strong vs Weak Acids
Acid strength is commonly assessed by the extent to which the acid dissociates in water. Strong acids dissociate nearly fully, while weak acids do not. However, no acid dissociates 100% completely in water.
There is debate about exact classifications since IUPAC has not set a strict cutoff for strong versus weak acids. Some chemists consider a compound with a pKa less than 0 as strong, while others use pKa < -1.7, the pKa of hydronium ion.
HClO3’s pKa and Its Implications
- HClO3’s pKa ≈ -1
- This value places it near the strong acid region
- It dissociates effectively in aqueous solutions to produce H+ and ClO3- ions
The near-complete dissociation means it behaves similarly to traditional strong acids like HCl and HNO3.
Practical Perspective on Acid Classification
Traditionally, there are seven strong acids recognized in chemistry, including HClO3. The distinction between strong and weak is often relative and contextual.
While some acids fall in a “borderline” pKa region, HClO3’s pKa firmly supports its strong acid classification, especially in most practical chemical scenarios.
Summary of Key Points
- HClO3 has a pKa around -1, consistent with strong acids.
- It dissociates almost completely in water, releasing protons efficiently.
- Definitions of acid strength vary, but HClO3 is generally accepted as strong.
- IUPAC does not enforce strict rules on acid strength cutoffs.
- In practical settings, HClO3 is treated as a strong acid.
Is HClO3 classified as a strong acid or a weak acid?
HClO3 is classified as a strong acid. It has a pKa of about -1, which means it dissociates almost completely in water to release protons.
What does the pKa value of HClO3 tell us about its acidity?
A pKa around -1 indicates that HClO3 loses its proton readily. This low pKa places it in the range of strong acids, which dissociate nearly fully in water.
Are definitions of strong and weak acids universally agreed upon?
No, there is no strict universal definition. Different sources use different pKa cutoffs; for example, some say pKa < 0 or pKa < -1.7 is a strong acid. IUPAC has not fixed a precise boundary.
Does HClO3 completely dissociate in water like the seven traditional strong acids?
HClO3 dissociates nearly completely in water. While “complete dissociation” is an ideal, HClO3 behaves similarly to the traditional strong acids in solution.
Why can the strong versus weak acid distinction be confusing or misleading?
The distinction depends on arbitrary cutoffs and definitions. Some acids fall in a borderline region of pKa values, making their classification flexible depending on context.




Leave a Comment