Why Does Krypton Have 2 Valence (Bonding Capacity) But 8 Valence Electrons?
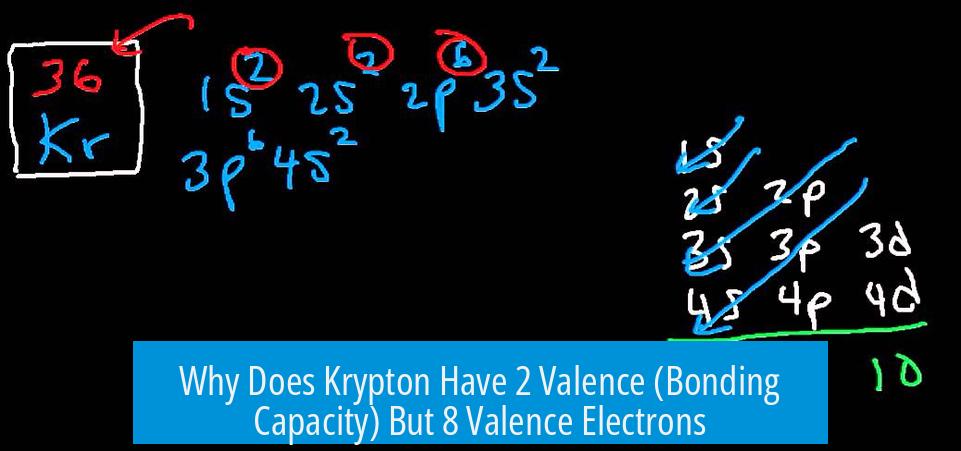
Krypton’s bottom number “2” indicates its valence or bonding capacity, not the count of valence electrons. Although krypton has 8 valence electrons in its outer shell, it typically forms up to 2 chemical bonds under exceptional conditions.
Distinguishing Valence Electrons from Valence
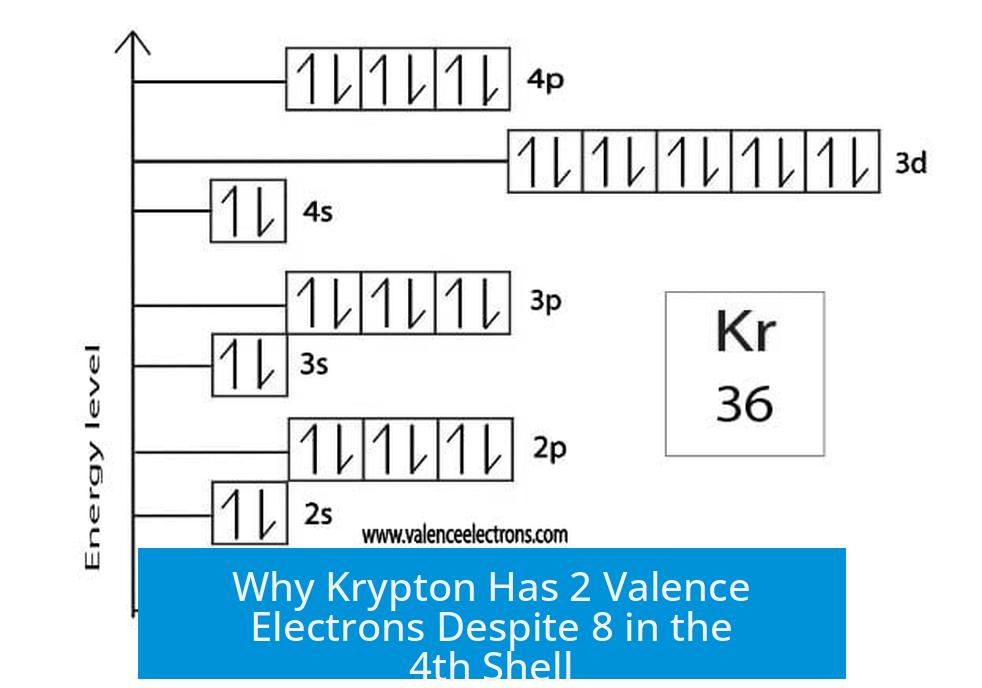
Krypton’s electron configuration shows 8 electrons in the 4th shell (4s2 4p6). These electrons are the valence electrons, the outermost electrons involved in chemical properties.
However, the valence number listed as “2” under the element symbol refers to how many bonds krypton usually forms, not how many valence electrons it has.
Electron Configuration and Stability
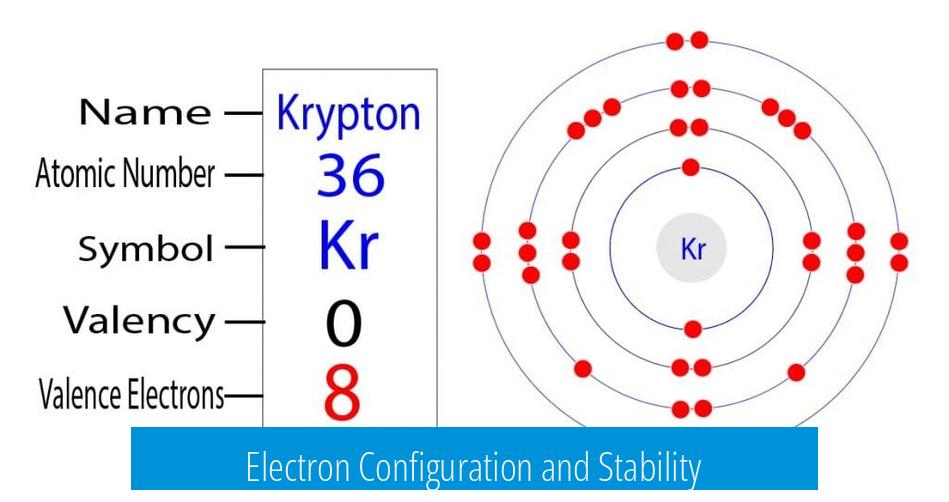
- Krypton’s outer shell is fully filled with 8 valence electrons, creating a stable noble gas electron arrangement.
- This full shell contributes to krypton’s low reactivity and chemical inertness under normal conditions.
- The 8 valence electrons fill the 4p orbitals, achieving an octet that resists forming bonds.
Why Valence is 2
The valence number indicates bonding capacity rather than electron count.
For krypton, the valence of 2 suggests it can form up to two bonds in unusual or forced chemical environments.
For example, krypton difluoride (KrF2) is a known compound where krypton forms bonds, but only under specific, high-energy conditions.
Summary of Key Points
- Valence electrons: Krypton has 8 electrons in its outermost shell (4th shell).
- Valence (bonding capacity): The number “2” indicates krypton can form up to two bonds, not the number of valence electrons.
- Full outer shell leads to chemical stability and an inert nature.
- Krypton’s bonding tendency is minimal and typically requires extreme conditions to form compounds.
Why does krypton show a valence of 2 when it has 8 valence electrons?
The number 2 refers to its bonding capacity, not how many valence electrons it has. Krypton can typically form only two bonds, even though it has 8 electrons in its outer shell.
How can krypton have 8 valence electrons but a valence of 2?
Krypton’s outer shell is full with 8 electrons, making it stable. The valence of 2 means it can form a maximum of two bonds, not the total electrons in the shell.
What causes krypton to have a low bonding capacity despite a full outer shell?
Krypton’s full 4th shell means it is chemically inert. This full shell prevents it from easily forming bonds, limiting its bonding capacity to two under special conditions.
Can krypton form bonds despite being stable with 8 valence electrons?
Yes, krypton can form compounds like KrF2 but only under forced conditions. This shows its valence of 2 reflects possible bonding, not typical behavior.
Is the bottom number under krypton’s symbol showing valence electrons or bonding capacity?
It shows valence, meaning the number of bonds krypton can form, not the count of valence electrons. The electrons in the outer shell are counted separately.




Leave a Comment