Electron configurations are an incredibly important concept in Chemistry, as they give us a way to understand the arrangement and behavior of electrons in different elements. A basic understanding of electron configurations helps us to understand the properties and behavior of different elements, and is a fundamental part of the Periodic Table.
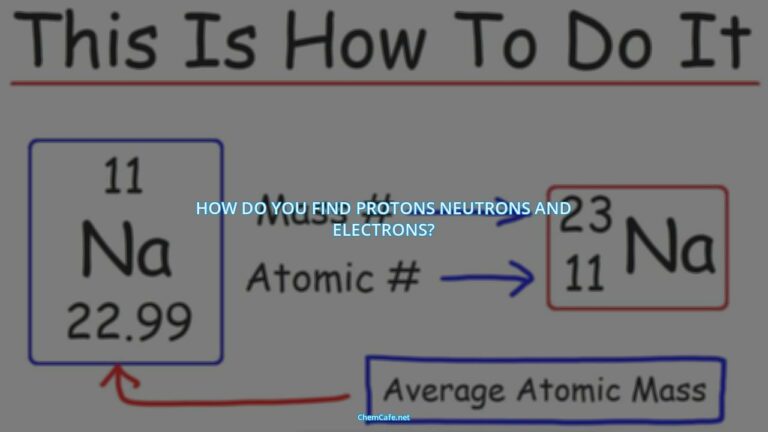
The electron configuration of an element is expressed in a standard notation, where all electron-containing atomic subshells are arranged in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1. But, for elements with larger atomic numbers, the electron configuration can become quite lengthy. In the case of the representative elements, the distinguishing electron will be in an ns or np subshell. This value of n, the principal quantum number for the distinguishing electron, can be determined by counting down from the top of the periodic table.
In particular, the electron configuration 1s22s22p63s23p64s13d10 corresponds to the element Pr, or praseodymium. The atoms of elements in the same vertical column of the periodic table will also have similar electron configurations. For example, the alkaline-earth elements (Group IIA) all have the same electron configuration, 1s22s22p63s2.
So, electron configurations are an incredibly useful tool in understanding the behavior of different elements, and how they interact with one another. Knowing the electron configuration of an element can help us to better understand its reactivity, and can provide insight into its physical and chemical properties. With a better understanding of electron configurations, we can better understand the structure and behavior of elements and the Periodic Table.
Which element corresponds to the electron configuration 1s22s22p63s23p64s13d10?
When it comes to understanding elements and their electron configurations, it can be helpful to refer to the periodic table. Electron configurations are the arrangement of electrons around the nucleus of an atom, and they can be used to identify which element an atom belongs to. The electron configuration 1s22s22p63s23p64s13d10 corresponds to the element Praseodymium, a rare earth element located in the 5th period of the periodic table.
What are Electron Configurations?
Electron configurations are used to describe the arrangement of electrons around the nucleus of an atom. These configurations are written using a standard notation which specifies the number of electrons in each of the atomic orbitals. For example, the electron configuration of sodium is written as 1s22s22p63s1. However, the standard notation often yields lengthy electron configurations, especially for elements with a large atomic number.
How to Identify the Element from an Electron Configuration?
In order to identify the element from a given electron configuration, it is helpful to refer to the periodic table. As a general rule, the distinguishing electron will be in an ns or np subshell. The value of n, the principal quantum number for the distinguishing electron, can be quickly determined by counting down from the top of the periodic table. For example, iodine is a representative element in the fifth period, so the distinguishing electron must occupy either the 5s or 5p subshell.
The electron configuration 1s22s22p63s23p64s13d10 corresponds to the element Praseodymium, a rare earth element located in the 5th period of the periodic table. This element is a soft, silvery white metal which is highly reactive and flammable in air.
The Relationship between Electron Configuration and the Periodic Table
It’s important to note that atoms of elements in the same vertical column of the periodic table have similar electron configurations. For example, the alkaline-earth elements (group IIA) all have the same electron configuration: 1s22s22p63s2. This is because the outermost electrons of these elements occupy the same subshells.
Electron configuration is an important concept in chemistry which can be used to identify elements and their properties. By referring to the periodic table, it is possible to quickly determine the element associated with a given electron configuration. In this article, we have discussed the electron configuration 1s22s22p63s23p64s13d10 and identified the element it corresponds to: Praseodymium, a rare earth element located in the 5th period of the periodic table.
What element is represented by the electron configuration 1s22s22p63s23p64s13d5?
The electron configuration 1s22s22p63s23p64s13d5 is the electron configuration of the element iron (Fe). Iron is one of the most abundant elements in the universe, and is a key component in many compounds. Its electron configuration gives us insight into the properties and reactivity of iron, and helps to understand why it is so important.
What are Electron Configurations?
Electron configurations are a way of representing the arrangement of electrons in an atom. They are written in a standard notation, with each electron-containing atomic subshell written in superscript. For example, the electron configuration of sodium is 1s22s22p63s1.
The Aufbau Principle states that electrons will occupy orbitals with lower energies before occupying orbitals with higher energies. This principle helps to explain why the electron configuration of iron (1s22s22p63s23p64s13d5) is the way it is.
Why are Electron Configurations Important?
Electron configurations are important for a variety of reasons. For example, they can be used to determine the valency of an element (the number of electrons it has available to form chemical bonds). They can also be used to predict the properties of a group of elements—elements with similar electron configurations tend to exhibit similar properties. Finally, electron configurations are helpful for interpreting atomic spectra.
The electron configuration 1s22s22p63s23p64s13d5 is the electron configuration of iron (Fe). Electron configurations are a way of representing the arrangement of electrons in an atom and are important for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. The Aufbau Principle explains why the electron configuration of iron is the way it is.
Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 quizlet?
Electron configurations are used to determine the valency of an element, predict the properties of a group of elements, and interpret atomic spectra. This notation for the distribution of electrons in the atomic orbitals of atoms was first used shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in 1913. But what exactly are electron configurations?
What are Electron Configurations?
Electron configurations describe how electrons are distributed in atomic orbitals. According to the standard notation, all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
However, the standard notation often yields lengthy electron configurations (especially for elements having a relatively large atomic number). For example, the electron configuration of chromium is 1s22s22p63s23p63d44s2. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written (here is an explanation why).
Half-filled and Fully Filled Subshells Have Extra Stability
Half-filled and fully filled subshells have extra stability. This means that when an atom has a subshell that is half-filled or completely filled, the electrons in that subshell are less likely to be involved in chemical reactions. This is why elements with similar electron configurations tend to exhibit similar properties.
Therefore, the element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 quizlet is chromium (Cr). This electron configuration is associated with extra stability, meaning that atoms of this element are less likely to be involved in chemical reactions. This is why chromium and other elements with similar electron configurations tend to exhibit similar properties.
In conclusion, electron configurations are useful for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. The element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 quizlet is chromium (Cr), which has extra stability due to its half-filled and fully filled subshells.
Which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1?
Electron configurations are an important part of understanding the behavior of atoms and molecules. Knowing the electron configuration of an atom can help us to predict its reactivity, its properties and its stability. It can also help us to understand how atoms interact with each other. In this blog, we will explore the electron configuration of elements and answer the question: which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1?
What is an Electron Configuration?
An electron configuration is a notation that describes the arrangement of electrons in an atom or molecule. It is written using the noble gas abbreviation, followed by the number of electrons in each subshell. For example, the electron configuration of sodium is 1s22s22p63s1. This means that there are two electrons in the 1s orbital, two in the 2s orbital, six in the 2p orbital, and one in the 3s orbital.
What are the Electron Configurations of the Elements?
The electron configurations of elements follow a pattern that is determined by the number of electrons in the atom. For elements with a relatively small atomic number, such as sodium (atomic number 11), the electron configuration is relatively straightforward. The electron configuration of sodium is 1s22s22p63s1.
For elements with a higher atomic number, such as silicon (atomic number 14), the electron configuration is longer and more complex. The electron configuration of silicon is 1s2 2s2 2p6 3s2 3p2.
Which of the Following Elements has the Electron Configuration 1s2 2s2 2p6 3s2 3p1?
The electron configuration 1s2 2s2 2p6 3s2 3p1 belongs to the element magnesium (Mg), which has an atomic number of 12. This means that there are two electrons in the 1s orbital, two in the 2s orbital, six in the 2p orbital, two in the 3s orbital, and one in the 3p orbital.
The other elements mentioned do not have this electron configuration. Sodium (Na) has an atomic number of 11 and its electron configuration is 1s22s22p63s1. Neon (Ne) has an atomic number of 10 and its electron configuration is 1s2 2s2 2p6. Aluminum (Al) has an atomic number of 13 and its electron configuration is 1s2 2s2 2p6 3s2 3p1.
In conclusion, the element which has the electron configuration 1s2 2s2 2p6 3s2 3p1 is magnesium (Mg). Electron configurations are an important part of understanding the behavior of atoms and molecules. Knowing the electron configuration of an atom can help us to predict its reactivity, its properties and its stability.
What element has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1?
Electron configurations allow us to understand the structure of atoms and predict the properties of elements. In a standard notation, all electron-containing atomic subshells are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1. But what element has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1?
The Answer: Chromium
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 is chromium. Chromium is an element of the transition metals, located in period 4 and group 6 of the periodic table. It has an atomic number of 24, an atomic mass of 51.996, and is represented by the symbol Cr.
What is electron configuration?
Electron configuration is the arrangement of electrons in an atom or molecule. It is expressed using the standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
Why is electron configuration important?
Electron configurations are important for a variety of reasons. Knowing the electron configuration of an element can be used to determine its valency, predict the properties of a group of elements, and interpret atomic spectra. Electron configurations can also be used to explain the behavior of elements in chemical reactions.
What is the shorthand electron configuration for chromium?
The electron configuration for chromium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1. The shorthand version of this is [Ar] 3d10 4s2 4p6, since argon’s electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6.
In conclusion, the element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 is chromium. Electron configurations are important for predicting the properties of elements and understanding the behavior of atoms in chemical reactions. The shorthand electron configuration for chromium is [Ar] 3d10 4s2 4p6.
What element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
When it comes to understanding the chemical behavior of elements, knowing their electron configurations is key. Electron configurations help us to understand what atoms are made of, how they interact with each other, and how they form molecules. So, what element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
The answer is copper. Copper is a transition metal with an atomic number of 29 and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. This means that the element has two electrons in its first shell (1s2), two electrons in its second shell (2s2), six electrons in its third shell (2p6), two electrons in its fourth shell (3s2), six electrons in its fifth shell (3p6), two electrons in its sixth shell (4s2), ten electrons in its seventh shell (3d10), and three electrons in its eighth shell (4p3).
Notation
The electron configuration of an atom is written with the help of subshell labels. These labels contain the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number) and the total number of electrons in the subshell in superscript. For example, if two electrons are filled in the ‘s’ subshell of the first shell, the resulting notation is ‘1s2’.
Uses of Electron Configurations
Electron Configurations are useful for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. By understanding the distribution of electrons in the atomic orbitals of an atom, researchers can determine the chemical and physical properties of the element.
Bohr Model of the Atom
The notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913. The Bohr model of the atom showed that electrons are arranged in shells around the nucleus, and each shell can hold a certain number of electrons. This model was later revised by Wolfgang Pauli, who proposed that electrons occupy orbitals of different shapes and orientations, which explains why some elements have more than one electron configuration.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 belongs to the element copper, a transition metal with an atomic number of 29. This electron configuration is written with the help of subshell labels and can be used to determine the valency of the element, predict the properties of a group of elements, and interpret atomic spectra. The notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented in 1913.



Leave a Comment