LiAlH4 vs. NaBH4 as a Reducing Agent
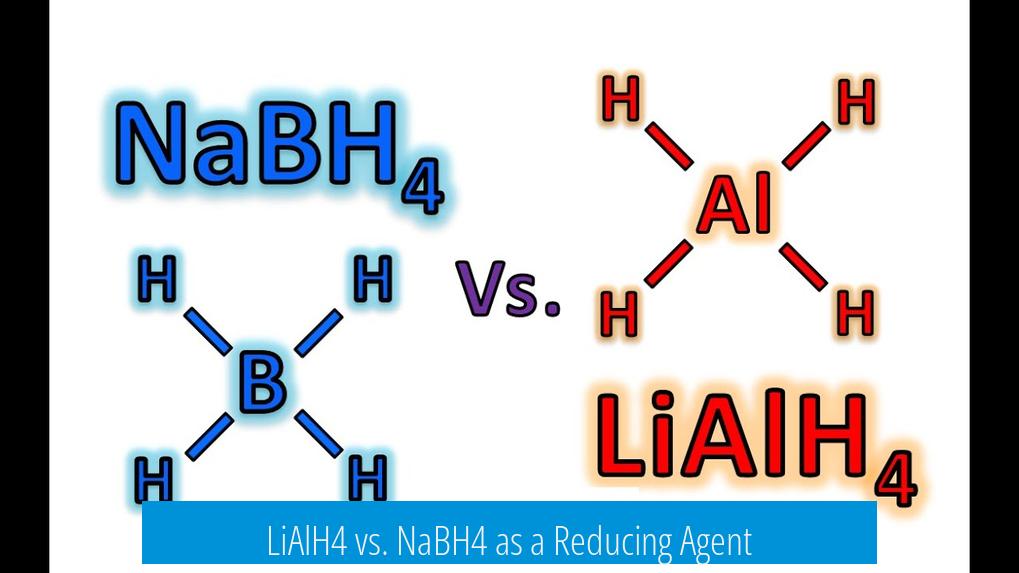
LiAlH4 (lithium aluminium hydride) is a stronger, more reactive reducing agent than NaBH4 (sodium borohydride). It can reduce a broader range of functional groups, including esters, carboxylic acids, and acid amides, whereas NaBH4 mainly reduces aldehydes and imines.
Strength and Reactivity
LiAlH4 acts as a powerful hydride donor, often called the “elephant gun” in organic chemistry due to its ability to reduce nearly all types of carbonyl compounds. This strong reactivity makes it suitable for challenging reductions that NaBH4 cannot achieve.
NaBH4 shows milder reactivity. It selectively reduces less resistant functional groups such as aldehydes and imines. It lacks the strength to reduce esters, acids, or acid amides effectively.
Solvent and Handling Requirements
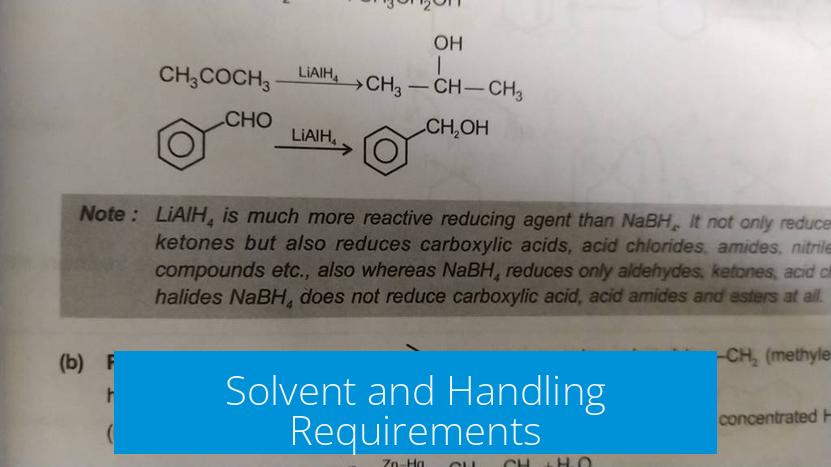
LiAlH4 requires strict anhydrous (dry) conditions. Contact with water or protic solvents can cause violent reactions, so it demands careful handling under inert atmosphere or dry solvents. This limits its practical use in many standard laboratory protocols.
NaBH4 tolerates protic solvents like methanol or ethanol and can be used directly from commercial solutions. This solvent compatibility increases its convenience and safety during routine reductions.
Substrate Scope and Selectivity
| Reducing Agent | Typical Substrates | Limitations |
|---|---|---|
| LiAlH4 | Esters, carboxylic acids, acid amides, aldehydes, ketones | Requires dry conditions, reacts violently with water |
| NaBH4 | Aldehydes, imines, some ketones | Cannot reduce esters, acids, acid amides; limited range |
LiAlH4 offers broad substrate compatibility, reducing functional groups that resist NaBH4. However, the choice between them often depends on the reduction target and practical considerations like solvent and safety.
Rate and Practical Considerations
LiAlH4 generally reduces substrates faster but requires controlled conditions. NaBH4’s milder nature often results in slower reductions but safer protocols. Chemists choose between these reagents based on the balance of reactivity, selectivity, and ease of handling.
Key Takeaways
- LiAlH4 is stronger and reduces broad functional groups including esters and acids.
- NaBH4 is milder and mainly reduces aldehydes and imines.
- LiAlH4 needs dry, aprotic solvents and careful handling.
- NaBH4 tolerates protic solvents like methanol.
- Choice depends on substrate, required reactivity, and handling safety.
What types of compounds can LiAlH4 reduce that NaBH4 cannot?
LiAlH4 reduces esters, carboxylic acids, and acid amides. NaBH4 usually can’t reduce these. It mainly works on aldehydes and imines.
Why must LiAlH4 be used under dry conditions?
LiAlH4 reacts strongly with water and protic solvents. Using it in dry conditions prevents decomposition and ensures it performs its reduction properly.
Can NaBH4 be used directly in methanol? Why?
Yes, NaBH4 is stable in methanol. This allows it to reduce compounds in protic solvents without special drying or precautions.
Which reducing agent is considered stronger and more reactive? Why?
LiAlH4 is stronger. It reduces a wider range of compounds and works faster but requires careful handling. NaBH4 is milder and less reactive.
How does substrate selectivity differ between LiAlH4 and NaBH4?
LiAlH4 reduces many functional groups, including tough ones like esters and acids. NaBH4 is selective for less reactive groups like aldehydes and imines.




Leave a Comment