Understanding the Difference Between Ground State and Excited Electrons
The difference between ground state and excited electrons lies in their energy levels and orbital positions within an atom. The ground state describes an electron at its lowest possible energy, while the excited state involves an electron elevated to a higher energy orbital after absorbing extra energy.
1. Defining Ground State and Excited State Electrons
The ground state refers to the condition when an electron occupies the lowest energy level available. This is the electron’s most stable arrangement. You can think of it as a ball resting at the bottom of a hill—holding the minimum potential energy possible.
In contrast, an excited state occurs when an electron absorbs energy and jumps to a higher energy level or orbital. Now, the ball has moved higher up the hill or is rolling somewhere on its slope. The electron has more energy than in the ground state but remains bound to the atom for the moment.
2. Classical vs. Quantum Mechanical Energy
Classical physics depicts energy as a continuous variable. For example, lifting an object increases its gravitational potential energy gradually, and it can take any value depending on height. Energy changes smoothly without jumps.
For electrons, the story is different. Quantum mechanics teaches that electrons do not possess energy values in a continuous range. Instead, electron energies are quantized, meaning they exist only at specific levels or “steps” of energy.
| Energy Type | Characteristic |
|---|---|
| Classical Energy | Continuous values, any amount is possible depending on position |
| Electron Energy (Quantum) | Discrete energy levels, only certain values allowed (quantized) |
3. Electron Energy Levels are Quantized
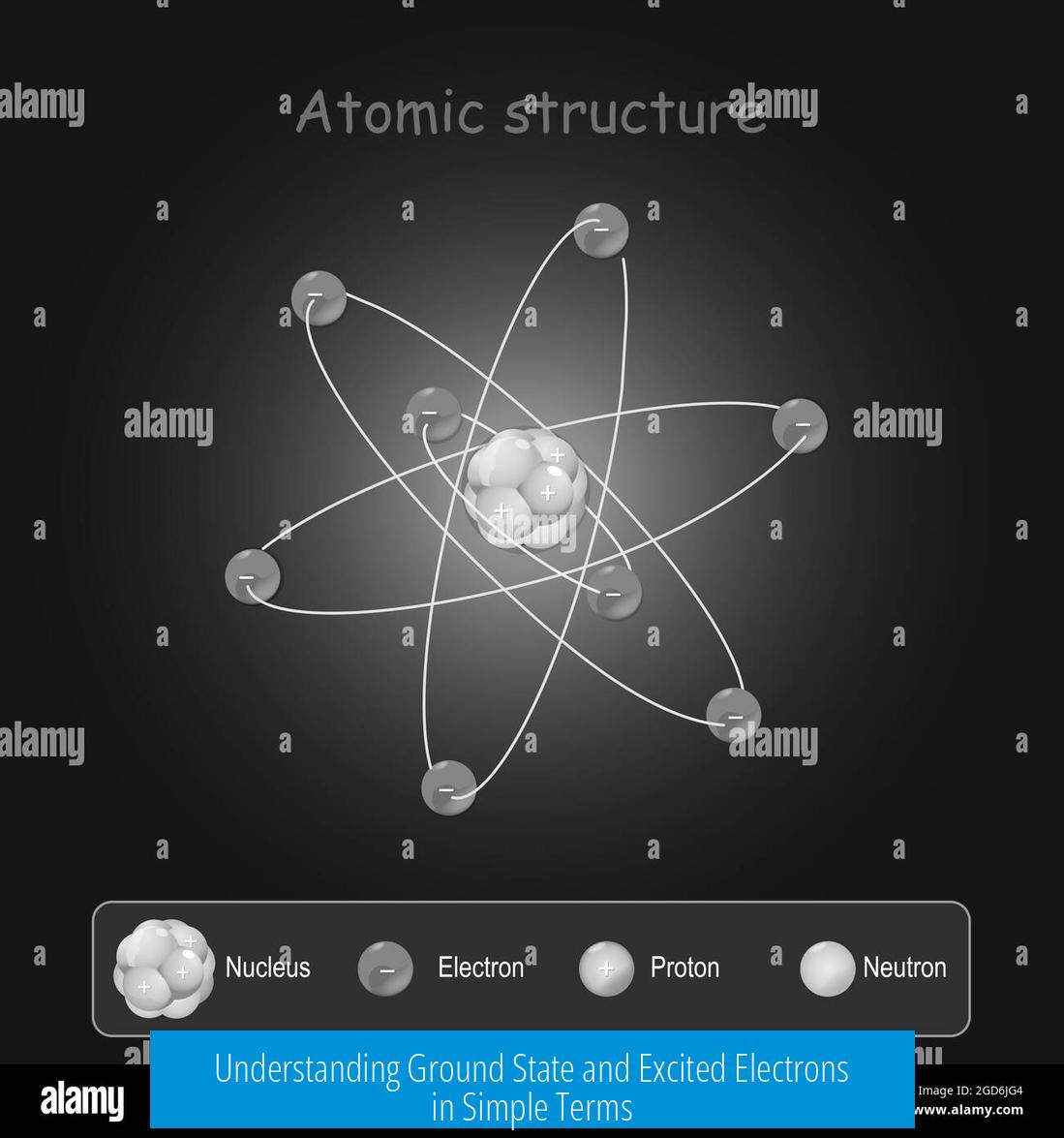
Electrons orbit the atomic nucleus but only at specific allowed distances and energy levels. These energy levels correspond to orbitals that the electron can occupy. The closest orbital to the nucleus is the ground state.
Electrons cannot exist in between these allowed orbitals, similar to how a staircase has distinct steps but no intermediate surfaces. This jump-like behavior contrasts with how an object can be at any height in classical terms.
4. Transition Between Ground and Excited States
To move from ground to excited state, an electron must absorb an exact quantity of energy corresponding to the gap between those energy levels. This energy is often supplied by absorbing a photon—an individual light particle—with energy tuned to that difference.
If the photon energy matches perfectly, the electron “jumps” to the higher orbital. If the photon energy is too low or too high, it generally will not be absorbed, and the electron remains in its original state.
5. Returning to the Ground State and Emission of Light
Electrons do not remain in excited states indefinitely. They tend to return to the ground state or a lower energy state by releasing the extra energy gained. This energy is emitted as a photon whose energy equals the difference between the two levels involved.
This emission is the basis of phenomena such as fluorescence, phosphorescence, and atomic emission spectra.
6. Analogies to Clarify Electron States
- Hill and Ball Analogy: The ground state is at the bottom of the hill (lowest potential energy). Excited states correspond to positions higher up the hill.
- Solar System-Orbital Analogy: Electrons orbit the nucleus like planets orbit the sun. The innermost orbit is the ground state. When excited, electrons move outward to higher orbits like planets moving further away.
These analogies help visualize discrete electron energies and the jump-like transitions caused by energy absorption or emission.
7. Detailed Look at Orbital Distances and Photon Interaction
Electrons orbit the nucleus at set distances defining energy levels. Orbitals closer to the nucleus hold less energy (ground state). Higher orbitals are farther out with greater energy.
When a photon strikes an electron with energy exactly matching the energy difference between its current and next orbital, the electron absorbs the photon and transitions to the excited state.
On returning to a lower orbital, the electron emits a photon with energy equal to the previous absorption, conserving energy precisely.
8. Energy Example and Photon Role
| Process | Energy Aspect | Photon Role |
|---|---|---|
| Ground to Excited State | Electron gains discrete energy equal to orbital gap | Photon with matching energy is absorbed |
| Excited to Ground State | Electron loses precise energy amount | Photon with same energy is emitted |
9. Implications for Atomic Behavior and Spectroscopy
The quantized nature of electron energies explains atomic line spectra—unique sets of wavelengths of emitted or absorbed light. Each element has characteristic energy gaps producing unique spectral lines.
This property enables techniques like flame tests, spectroscopy, and lasers, all relying on electrons moving between ground and excited states.
Summary of Key Points
- Ground state electrons occupy the lowest energy orbital, the most stable condition.
- Excited state electrons have absorbed energy and occupy higher orbitals temporarily.
- Electron energies are quantized, existing in fixed, discrete levels only.
- Photon absorption causes electrons to jump from ground to excited states.
- Photon emission occurs when electrons return from excited states to ground or lower states.
- Electrons cannot exist between quantized orbitals, unlike classical continuous energy.
- Hill and solar system analogies help visualize energy states and transitions.
- Atomic behavior such as emission spectra depends on these quantized electron transitions.



Leave a Comment