What Are Orbitals? A Simple Explanation
Orbitals are regions around an atom where electrons are most likely to be found at any given time. They explain why atoms bond, how bonds form, and influence chemical properties like color and reactivity.
Orbitals are not mere drawings or symbols; they represent real, physical probability distributions describing where electrons exist. They do not depict fixed paths but rather “fuzzy” zones shaped by quantum behaviors.
Why Orbitals Matter in Chemistry
Orbitals control about 90% of chemistry. They dictate if atoms bond and what kind of bonds they form. The shape and energy of orbitals influence molecular geometry, stability, and interactions.
Understanding orbitals clarifies:
- Why some elements form compounds and others do not
- The types of chemical bonds (e.g., single, double)
- Color and energy absorption in molecules
Electrons Are Hard to Pinpoint
Electrons are extremely small and travel fast. It is impossible to know their exact place at any moment. Instead, orbitals show where electrons probably are.
The shape of an orbital is the area where there is about a 97% chance of finding an electron. This shape depends on the electron’s energy, angular momentum, and other quantum properties.
Probability Distribution of Orbitals
- Orbitals describe probability, not fixed paths.
- They reflect the wave nature of electrons.
- The electron’s exact location varies, but its probability cloud stays consistent.
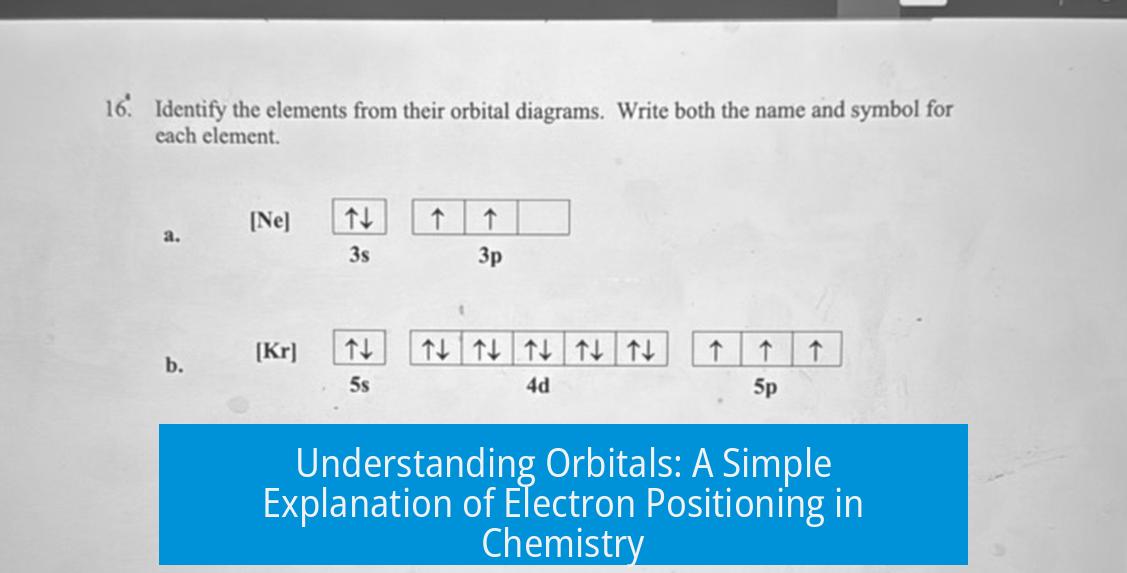
Shapes and Types of Orbitals
Orbitals vary in shape and energy. These shapes correspond to electron energy levels and spatial orientation. They come in groups called s, p, d, and f orbitals.
| Orbital Type | Shape | Electron Capacity |
|---|---|---|
| s | Sphere | 2 electrons |
| p | Dumbbell | 6 electrons |
| d | Complex cloverleaf | 10 electrons |
| f | More complex shapes | 14 electrons |
As you move down the periodic table, orbitals increase in energy and complexity to hold more electrons.
Visualizing Orbitals with Analogies
Analogies help make orbitals easier to grasp:
- Egg Cartons: Think of orbitals like egg cartons with limited spots. When full, electrons stay put; when empty, electrons can share or bond with other atoms.
- Waves: Imagine ocean waves instead of tiny points. The height and spread of a wave reflect where electrons likely reside, not an exact path.
These analogies emphasize that orbitals are fuzzy clouds where electrons hover with a good chance, not tiny planets orbiting the nucleus.
Wave-Particle Duality and Why Orbitals Are Preferred
Electrons behave as both particles and waves. For chemistry, treating them as waves is more accurate. Electron waves create orbital shapes.
The older Bohr model, where electrons circle the nucleus like planets, is outdated. Electrons cannot be pinpoint particles with zero volume; this contradicts physics and requires infinite energy to confine.
Orbitals reflect the electrons’ wave nature, showing probable regions rather than paths.
Summary of Orbitals in Electron Structure
- Orbitals represent the space where electrons live around the nucleus.
- They are probability clouds, not fixed orbits.
- Shapes vary by type (s, p, d, f) and energy levels.
- Orbitals explain chemical bonding and many atomic properties.
- Electron position is described by probability, not certainty.
- Wave nature of electrons underpins the orbital model.
Key Takeaways
- Orbitals show regions of high likelihood of finding an electron.
- They determine atomic behavior and chemical bonding.
- Orbitals have distinct shapes and energies depending on electron properties.
- The electron’s wave-like behavior explains orbital shapes.
- Analogies like egg cartons and waves help visualize orbitals.



Leave a Comment