Difference between a Polar Covalent Bond and Ionic Bond
A polar covalent bond involves unequal sharing of electrons between nonmetal atoms, while an ionic bond arises from the complete transfer of electrons between a metal and a nonmetal, forming charged ions that attract each other.
Nature of Bonding
Ionic Bonding
Ionic bonds form between metal and nonmetal atoms. In this process, atoms become ions by fully gaining or losing electrons to attain stability.
These ions carry positive or negative charges, similar to magnets, and the electrostatic attraction between them creates the ionic bond.
- Example: NaCl – sodium (metal) loses an electron becoming Na+, chlorine (nonmetal) gains one becoming Cl−.
- Bond formed by attraction of opposite charges.
Covalent Bonding
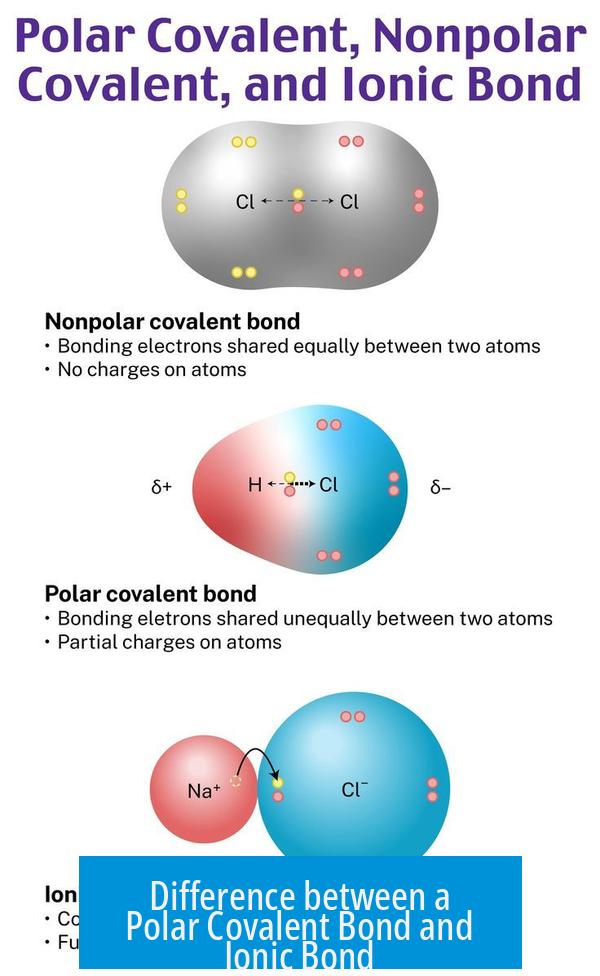
Covalent bonds occur between nonmetal atoms sharing electrons to fill their outer shells.
Electrons are shared rather than transferred, like two atoms “borrowing” electrons.
- Sharing may be equal (nonpolar) or unequal (polar).
- Number of electrons involved depends on the atoms’ needs to complete octets.
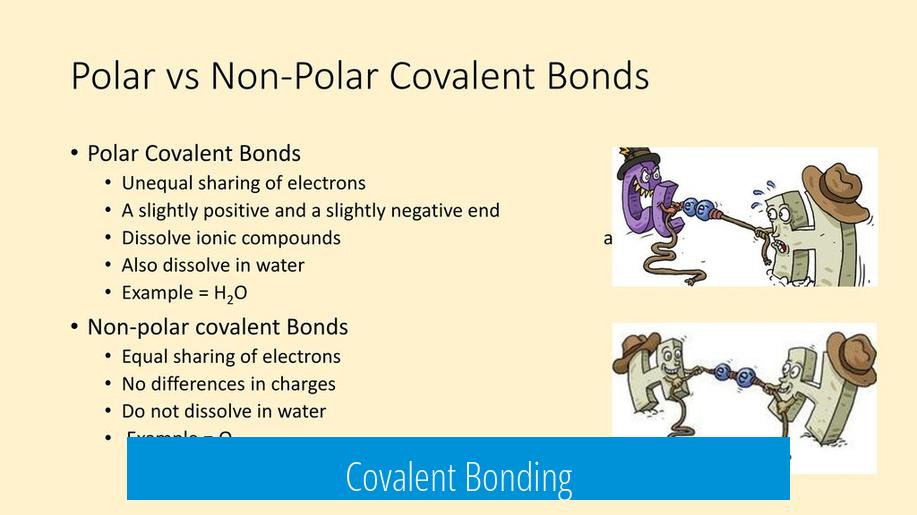
Polar Covalent Bonding
Polar covalent bonds represent unequal sharing of electrons between two nonmetal atoms due to differences in electronegativity.
One atom attracts the shared electrons more strongly, creating partial charges.
- Example: Hydrogen fluoride (HF) – fluorine pulls electrons more than hydrogen.
- Results in a dipole with partial positive and negative ends.
Electronegativity and Bond Spectrum
| Bond Type | Electronegativity Difference | Electron Behavior | Resulting Particles |
|---|---|---|---|
| Ionic Bond | Large (typically >1.7) | Complete transfer of electrons | Separate charged ions |
| Polar Covalent Bond | Moderate (0.4 to 1.7) | Unequal sharing of electrons | Partial charges (dipoles) |
| Nonpolar Covalent Bond | Small or zero (<0.4) | Equal sharing of electrons | No partial charges |
Bonding lies on a continuous scale. Ionic bonds exhibit full electron transfer. Polar covalent bonds show unequal sharing but electrons remain shared to some extent.
Key Takeaways
- Ionic bonds form between metals and nonmetals via electron transfer.
- Polar covalent bonds occur between nonmetals with unequal electron sharing.
- Electronegativity differences govern the bond type on a spectrum.
- Ionic bonds produce charged ions; polar covalent bonds create dipoles.
Difference Between a Polar Covalent Bond and Ionic Bond: The Science Behind the Sticky Situation
So, what’s the difference between a polar covalent bond and an ionic bond? The short answer is: ionic bonds are the result of a full transfer of electrons between atoms, creating charged ions that attract each other, while polar covalent bonds involve an unequal sharing of electrons between atoms. That might sound like chemistry jargon, but hang with me. We’ll break it down in a relatable way that makes these invisible forces stick in your mind.
The Bonding Basics: “Sharing” vs “Stealing” Electrons
Imagine atoms like kids with toys. In ionic bonding, one kid (a metal atom) basically takes a toy (electron) away from the other kid (a nonmetal atom). That nonmetal, now deprived but stable in its new arrangement, becomes negatively charged because it has gained an electron. Meanwhile, the metal becomes positively charged because it lost one. Charged particles? Check. Opposites attract? Double check. Boom—an ionic bond.
On the flip side, covalent bonds are more like kids who play nicely and share their toys instead of stealing. They hold onto the electrons together. Sometimes, it’s an equal share (nonpolar covalent bonds), which is like two kids holding the toy right in the middle. Other times, it’s unequal sharing, where one kid holds the toy more than the other—that’s where polar covalent bonds come in.
Polar Covalent Bonds: Unequal Sharing in Action
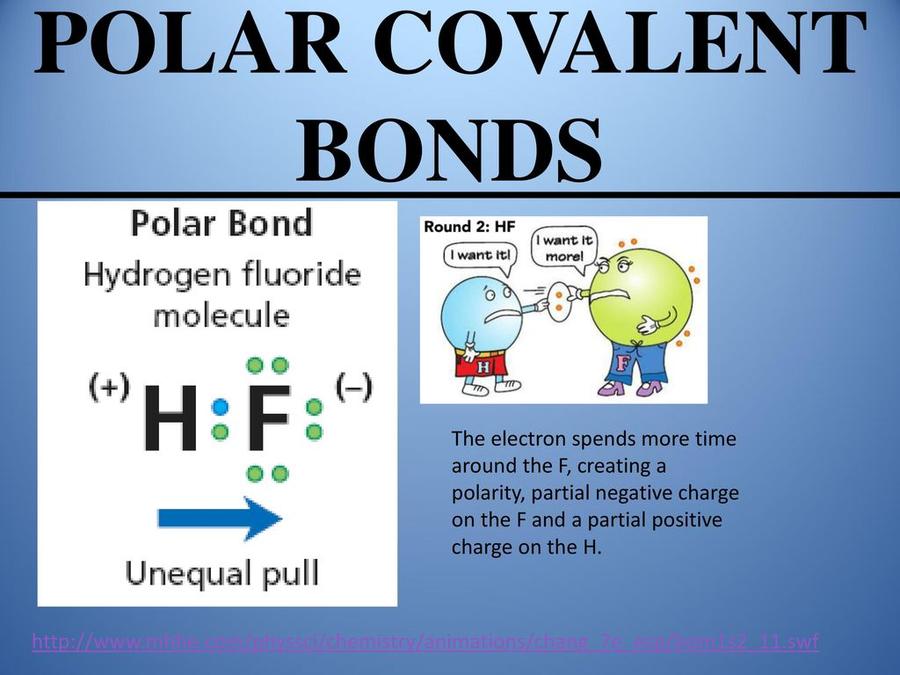
Take the example of hydrogen fluoride (HF). The fluorine atom is like the kid who’s got the biggest, best toy and a strong grip on it. Fluorine is highly electronegative, meaning it has a powerful pull on electrons. Hydrogen, although a nonmetal and a bit strange because it’s in group 1 on the periodic table (usually reserved for metals), shares the electron but not equally. The electron hangs out more around fluorine, causing a “polar” situation where one end is partially negative and the other partially positive. It’s like a tug-of-war where fluorine wins, but hydrogen still holds on just enough so there’s no actual electron theft.
Ionic Bonds: Charged Magnets Attract
In contrast to the “sharing” game, ionic bonding is straightforward. Metals love to ditch electrons to feel stable. Nonmetals adore snagging those extras. Once metals lose their electrons, they become positively charged cations; nonmetals gain electrons to become negatively charged anions. These opposite charges are like magnets—strongly pulling the ions together. That’s why table salt (NaCl), an iconic ionic compound, forms strong crystal lattices; the sodium ion (Na+) and chloride ion (Cl−) lock together tightly.
Electronegativity: The Bond Spectrum
Think of chemical bonds as existing along a spectrum defined by electronegativity, a term chemists use to describe an atom’s electron-grabbing power. At one end, you have equal electron sharing (nonpolar covalent). Moving along, you encounter unequal sharing (polar covalent), and at the extreme end, full electron transfer happens, giving ionic bonds.
| Type of Bond | Electron Behavior | Atoms Involved | Resulting Charge |
|---|---|---|---|
| Nonpolar Covalent | Equal sharing | Nonmetal & nonmetal (similar electronegativity) | No charge |
| Polar Covalent | Unequal sharing | Nonmetal & nonmetal (different electronegativity) | Partial charges |
| Ionic | Full electron transfer | Metal & nonmetal | Full positive and negative ions |
Why Does This Matter? The Real-World Impact

You might think, “Okay, but why should I care about polar covalent vs ionic bonds?” Well, these bonds dictate everything from the way salt dissolves in water to how our DNA holds its structure.
For instance, ionic bonds form strong crystals that dissolve easily in water, making salty water taste so good and be critical for life’s functions. Meanwhile, polar covalent bonds create molecules like water itself (H2O), which has a slightly positive and negative end allowing unique properties like surface tension and solvent capabilities essential for life.
Making Sense of Bonding: Think of It Like Relationships
If atoms were people, ionic bonds would be the strict “I’m giving you this, and you must take it or else” kind of relationship, based purely on opposite charges attracting. Polar covalent bonds are more like a friendship with some power dynamics—you share, but not equally. Nonpolar covalent bonds? True equality and harmony.
Both types of bonds have their role. Ionic bonds are great for building strong, solid stuff that dissolves and behaves predictably. Polar covalent bonds make molecules that are flexible, reactive, and capable of building the complex macromolecules life depends on.
Want to Spot the Bond Type? Here’s a Quick Tip
- If you see a combo of metal and nonmetal? You’re probably looking at an ionic bond.
- Two nonmetals sharing electrons? Covalent bond territory.
- When nonmetals share but one pulls harder? Polar covalent bond.
Next time you’re holding a salt shaker or a water bottle, remember the hidden drama of electrons playing tug-of-war inside. Understanding these bonds isn’t just chemistry class fluff—it’s the story of how matter sticks together, in every little way.
So, next time someone asks about the difference between polar covalent and ionic bonds, you can confidently say: “Ions borrow and hold hands with charges, while polar covalent bonds share unevenly in electron playgrounds.”
And if you tell them hydrogen is a weird nonmetal in group 1? They’ll know you really mean business.
What defines an ionic bond compared to a polar covalent bond?
Ionic bonds form when a metal atom transfers electrons to a nonmetal, creating charged ions. These ions attract each other due to opposite charges. Polar covalent bonds share electrons unevenly between nonmetals due to electronegativity differences.
How does electronegativity influence polar covalent bonds?
Electronegativity difference causes unequal sharing of electrons in polar covalent bonds. For example, fluorine pulls electrons more strongly than hydrogen in HF, making the bond polar.
Do ionic bonds always involve full electron transfer?
Yes, ionic bonds result from a full transfer of electrons. This transfer creates charged ions that attract each other. It contrasts with polar covalent bonds, where electrons are shared but unevenly.
Can a bond be between two nonmetals and still ionic?
No, typically ionic bonds form between a metal and a nonmetal. Bonds between two nonmetals are usually covalent, either polar or nonpolar, depending on electron sharing.
What is the key difference in electron behavior in ionic vs polar covalent bonds?
Ionic bonds ‘steal’ electrons, creating ions bonded by charge attraction. Polar covalent bonds involve sharing electrons unequally but not full transfer, leading to partial charges on atoms.




Leave a Comment