How Can I Go About Determining the Atomic Number of Different Elements on My Own?
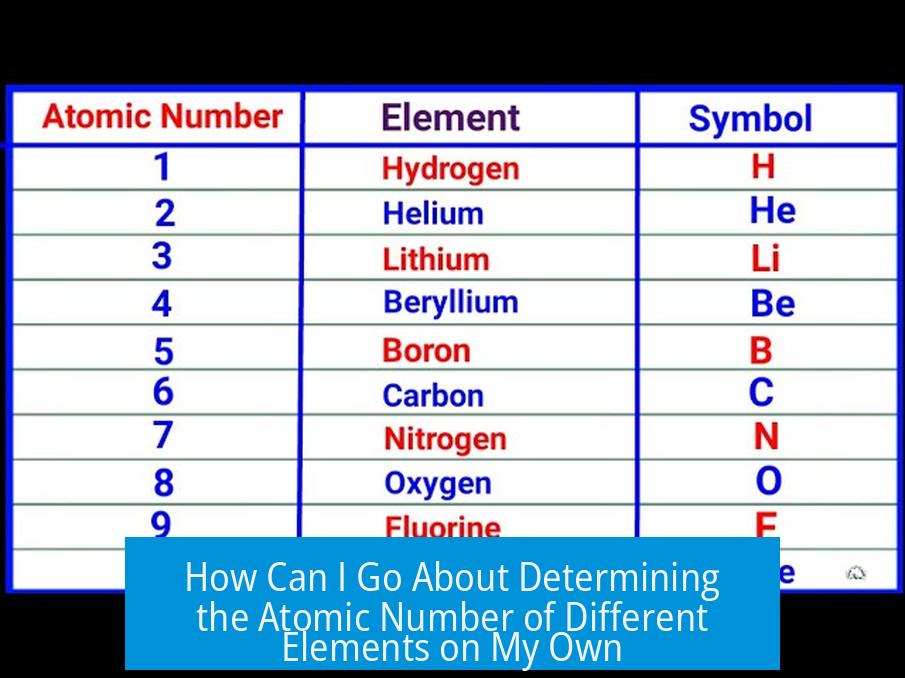
The atomic number is the number of protons in an element’s nucleus and can be determined by measuring the energy of characteristic X-rays emitted when atoms are exposed to X-rays and applying Moseley’s Law, which relates the X-ray frequencies to atomic number mathematically.
This answer covers key historical and modern methods, from early chemical techniques to precise spectroscopic measurements.
Understanding the Atomic Number
The atomic number (Z) is fundamental as it defines the identity of an element. Each element has a unique number of protons in its nucleus, which dictates its chemical properties and place in the periodic table.
Protons and Atomic Number
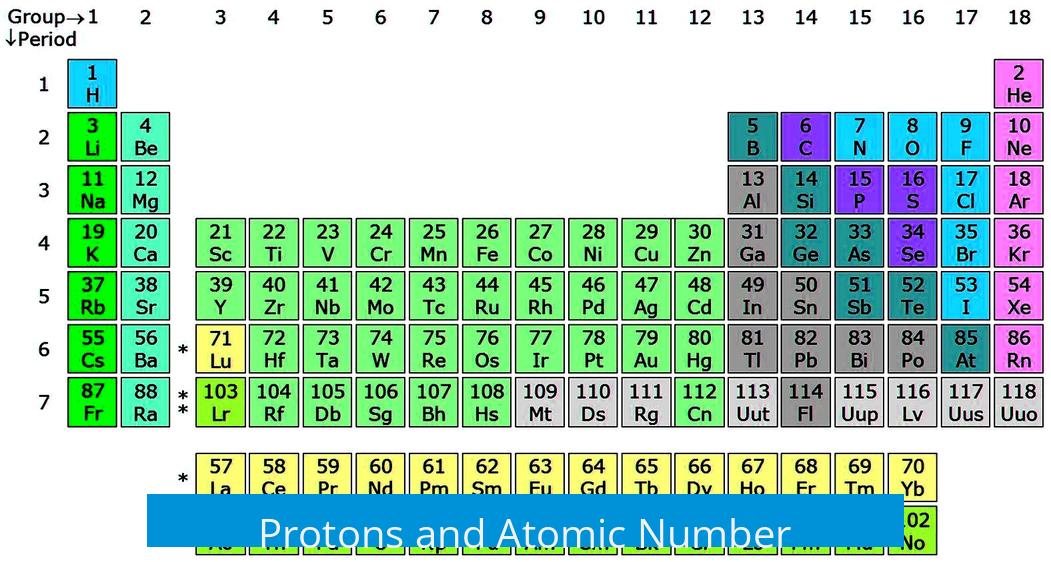
The atomic number equals the number of protons, a fact discovered well after early atomic models. Protons are positively charged particles in the nucleus, balancing the negatively charged electrons and determining the element’s identity.
Historical Approaches: From Atomic Weight to Atomic Number
Mendeleev’s Periodic Table and Atomic Weights
- Dmitri Mendeleev arranged elements by atomic weight, not atomic number, as protons had not been discovered in the 19th century.
- He organized the table to reflect chemical properties, even if it meant rearranging by weight inconsistencies.
- Atomic weight is roughly proportional to atomic number but has limitations in predicting chemical behavior precisely.
Using Chemical Stoichiometry to Estimate Atomic Mass Ratios
Before atomic number was known, chemists relied on the Law of Definite Proportions. This law states that chemical compounds always contain elements in fixed mass ratios.
For example, water is made of hydrogen and oxygen in an 1:8 mass ratio. Knowing the mass ratio and composition lets you estimate relative atomic masses.
| Element | Mass Ratio in Water | Approximate Atomic Mass (Relative to H) |
|---|---|---|
| Hydrogen (H) | 1 | 1 |
| Oxygen (O) | 8 | 16 (due to H2O having 2 H atoms) |
By conducting experiments like electrolysis to measure hydrogen and oxygen produced, one could calculate their atomic masses relative to one another.
Spectral Emission as Elemental Fingerprints
Early identification used flame tests or vaporizing samples to analyze the color of emitted light. Each element emits light at characteristic wavelengths when excited.
Such spectral lines act like unique signatures but initially only helped to identify elements, not directly to determine atomic number.
Moseley’s X-ray Experiments: Linking X-rays and Atomic Number
Inner-Shell Electron Transitions and X-ray Emissions
In 1913, Henry Moseley studied how metals emit X-rays after being hit with X-rays of their own. He observed that electrons in the innermost K-shell (1s orbital) can be ejected by irradiation.
This creates a vacancy, which outer shell electrons fill. When an electron falls into the vacancy, it emits an X-ray photon with specific energy.
The Energy Relation to Atomic Number
The energy of emitted X-rays, notably the Kα line, depends on the nuclear charge. Moseley found the energy was proportional to (Z – 1)2, where Z represents the atomic number.
This led to Moseley’s Law, expressed as:
E = k (Z – b)2
Where E is the X-ray frequency or energy, k and b are constants determined experimentally or theoretically.
Bohr Model and Screening Effects
Niels Bohr’s atomic model helped interpret these X-ray energies. Electrons partially screen the nuclear charge, reducing the effective charge felt by outer electrons.
For K-shell emissions:
- The effective charge is approximately Z – 1, due to one electron screening.
- For L-shell emissions, the screening constant differs (~7.4), altering the energy equation.
Moseley’s Plot and Discovering Atomic Number
By plotting the square root of measured X-ray frequencies against atomic numbers, Moseley found a linear relationship. Missing elements appeared as gaps in this plot.
This method allowed accurate assignments of atomic numbers independent of atomic mass, revealing the true order of elements.
Practical Steps to Determine Atomic Number Yourself
- Prepare thin metal foils of the element to study.
- Subject the sample to X-ray irradiation to eject inner-shell electrons.
- Use an X-ray spectrometer to detect emitted characteristic X-rays (such as Kα or Lα lines).
- Measure the frequency or energy of the X-rays emitted.
- Apply Moseley’s law equations, possibly involving the Bohr model, to solve for the atomic number Z.
This process requires precise instrumentation but is foundational in analytical chemistry and material science.
Modern Methods and Instrumentation
Advanced Spectroscopic Techniques
Today, instruments like mass spectrometers separate isotopes and elements precisely by mass-to-charge ratios.
- Nuclear Magnetic Resonance (NMR)
- Infrared Spectroscopy (IR)
- Ultraviolet-Visible Spectroscopy (UV-Vis)
- X-ray Fluorescence (XRF)
- Mass Spectrometry
These methods allow rapid, non-destructive elemental and isotopic identification.
Spectral Libraries and Databases
Full spectral databases exist cataloging emissions, absorption spectra, and mass data, providing references for known elements and their atomic numbers.
Comparing measured data against these databases can quickly determine the element and its atomic number indirectly.
Summary: Determining Atomic Number Techniques
- Early Era: Use chemical reaction mass ratios and stoichiometry to estimate relative atomic masses, which approximate atomic numbers.
- Spectral Emissions: Vaporize samples and record emission colors and wavelengths, aiding qualitative identification.
- X-ray Spectroscopy and Moseley’s Law: Measure X-ray photon energies emitted from electron transitions to calculate atomic numbers precisely.
- Modern Analytical Methods: Employ mass spectrometry and advanced spectroscopy for direct elemental and isotopic analysis.
Key Takeaways
- Atomic number equals the number of protons and defines elements uniquely.
- Mendeleev organized elements by atomic weight, but atomic number came later through X-ray studies.
- Moseley’s X-ray experiments provided a direct mathematical link between emitted X-ray energies and atomic number.
- Determining atomic number requires measurement of characteristic X-rays and application of Moseley’s law equations.
- Modern instruments offer fast, precise elemental analysis using spectroscopy and mass spectrometry.
How Can I Go About Determining the Atomic Number of Different Elements on My Own?
Answer first: You determine the atomic number of an element by measuring the frequency of X-rays it emits when its inner electrons get knocked out, then applying a precise formula developed from atomic physics. Sounds complex? Let’s break it down with some history, science, and real-world methods you can explore at home or in a lab.
Ever wondered why elements have a secret number that defines them? That’s the atomic number, which equals the number of protons in the nucleus. It’s like the element’s unique ID card. Figuring it out isn’t just academic; it tells you what makes one element different from another in chemical behavior. If you want to determine this number on your own, we’ll journey through discovery, from early ideas to modern techniques.
Understanding the Roots: Mendeleev and Atomic Weight Mix-Ups
Back in 1869, Dmitri Mendeleev shook the scientific world by arranging elements in a periodic table, but here’s the catch: he did not know about protons or atomic numbers. He ordered elements by atomic weight — basically how heavy each atom was compared to hydrogen.
Mendeleev noticed oddities. Sometimes, when he arranged elements strictly by atomic weight, their chemical properties didn’t line up as expected. He bet on chemistry being right and suspected the atomic weights might be off. Turns out, he was onto something—he was sorting without the real key: the number of protons, which no one had discovered yet.
So, if you’re back in the 19th century with just a scale and some chemicals, this is your starting point: measure atomic weights through chemical analysis and stoichiometry.
Early DIY Method: Stoichiometry and Chemical Analysis
Imagine you have a bottle of water and want to find out about oxygen’s weight relative to hydrogen. Chemists found that water is always made of 2 hydrogen atoms for every 1 oxygen atom, with oxygen weighing 16 times as much as hydrogen by atomic mass. They figured this out by carefully measuring the mass of gases released from water decomposition or how much oxygen was needed to react with hydrogen.
This method is hands-on and practical, but it only gives you atomic mass ratios, not atomic numbers. Plus, it takes patience and balance scales sensitive enough to detect small differences.
Getting Fancy: Identifying Elements by Their Light
Before anybody knew about protons, scientists relied on a beautiful trick: vaporize a sample and look at its light. Every element emits a unique emission spectrum, sort of like a fingerprint in light form.
By heating or electrifying a sample and analyzing the emitted light through a prism or diffraction grating, you see bright colored lines at specific wavelengths. Each line corresponds to one jump of an electron dropping to a lower energy state. This spectroscopy method—though it won’t tell you the atomic number outright—helps identify which element you have.
The Breakthrough: Moseley’s X-ray Diffraction Experiments
Enter Henry Moseley, the early 20th-century physics wizard who put a number on the atomic number problem. He used X-rays to knock out inner electrons from metal foils. When inner electron ‘holes’ appeared, outer electrons fell in to fill them, releasing X-rays whose energies closely related to the atomic number.
This process might sound like science fiction, but it’s real physics: Moseley discovered a formula connecting the energy of emitted X-rays to the square of Z – constant, where Z is the atomic number.
In simpler words: If you measure the X-ray energy emitted by an atom, you can calculate its atomic number by plugging the value into Moseley’s law.
The Bohr Model Makes It Possible
Moseley’s law builds on Niels Bohr’s model of the atom. Bohr pictured electrons orbiting the nucleus in energy levels. The energy difference when an electron jumps to fill an inner-shell vacancy defines the frequency of X-ray radiation emitted.
The formula varies slightly depending on which electron shell is involved, with values adjusted for the “screening effect”—the shielding from other electrons affecting nuclear charge felt by the electron being ejected.
How Could You Replicate This at Home or in a Simple Lab?
- Get an X-ray source and samples of metal foils. Not as easy as buying groceries, but research labs or educational facilities often have safe setups for this.
- Irradiate your sample with X-rays. This knocks inner-shell electrons out.
- Detect the emission spectrum of the emitted X-rays. Use an X-ray detector (usually a specialized spectrometer).
- Measure the frequency or energy of the emitted X-ray lines (Kα or Lα emissions).
- Apply Moseley’s formula: The energy relates to (Z – constant)^2. Rearrange to solve for Z, the atomic number.
Granted, this approach requires specialized equipment. But it’s the gold standard of determining atomic numbers.
Modern Marvels: Spectroscopy and Mass Spectrometry
Today, you don’t need to build X-ray setups yourself. Modern spectrometers can analyze materials instantly. Techniques like UV-Visible, IR, NMR, and Mass Spectrometry provide precise element identification and isotope differentiation.
Mass spectrometers, for instance, ionize atoms and measure mass-to-charge ratios, which can hint at atomic weights and help clarify atomic numbers indirectly by composition.
This is even better for mixtures or unknown compounds—as seen when testing gemstones like emeralds. The instruments identify and quantify all elements inside based on their unique spectra.
Why Does It Matter?
Knowing an element’s atomic number is foundational in chemistry and physics. Imagine designing new materials, studying star compositions, or diagnosing medical materials. The ability to determine atomic number yourself bridges the gap between raw curiosity and scientific discovery.
It’s a bit like solving a cosmic Sudoku puzzle with atomic clues. Understanding these methods gives you insight into the very building blocks of the universe.
Summary: Step-by-Step DIY Atomic Number Discovery
- Start by analyzing chemical reactions and stoichiometry to estimate relative atomic weights through mass measurements of compounds.
- Use emission spectroscopy by vaporizing your sample and observing unique light signatures to identify elements qualitatively.
- For early 20th-century level accuracy, irradiate your sample with X-rays, measure the emitted X-ray frequencies (K or L lines), and calculate the atomic number using Bohr’s model and Moseley’s law.
- Modern methods include sending your element through spectroscopy or mass spectroscopy machines for rapid and precise results.
Final Thought
While determining atomic numbers on your own requires sophisticated equipment that’s usually found in research labs, understanding the principles allows you to appreciate the triumphs of scientific discovery. Plus, you can replicate parts of the process at home using stoichiometry or visible spectroscopy with simpler tools.
Ever tried decomposing water to weigh hydrogen and oxygen? Or looking at emission lines through a prism? That’s practically a mini adventure into atomic science. So, are you ready to grab some goggles and your periodic table? Chemistry awaits your experiments!
Further Resources to Explore
- Henry Moseley and Moseley’s Law — Dive deeper into the physics that cracked atomic numbers wide open.
- Emission Spectrum — Explore how light reveals element identities.
- Bohr Model — Understand the atom’s energy levels behind these discoveries.
- Rutherford Scattering Experiment — Learn about the experiment that led to our knowledge of the atomic nucleus.
How can I use chemical reactions to estimate an element’s atomic number?
By measuring the mass ratios of elements in a compound and knowing the stoichiometry, you can find relative atomic weights. Comparing these to hydrogen’s mass helps estimate atomic weights, which relate closely to atomic numbers in many cases.
What role does X-ray spectroscopy play in finding atomic numbers?
When you irradiate an element with X-rays, inner electrons are ejected. Outer electrons fall into the hole, emitting X-rays with energies tied to the atomic number. Measuring these energies lets you calculate the atomic number using Moseley’s law and Bohr’s model.
Can I determine atomic number by just vaporizing a sample and observing spectral lines?
Vaporizing and analyzing spectral emission lines helps identify elements qualitatively. Each element emits unique wavelengths, but estimating atomic number accurately requires measuring X-ray emissions or advanced spectral techniques.
How does Moseley’s law help in determining atomic numbers?
Moseley’s law links the square root of X-ray emission energy to the atomic number. By measuring emitted X-ray frequency and applying this law, you can plot and identify atomic numbers even for unknown elements systematically.
Are modern tools like mass spectrometry useful for finding atomic numbers?
Yes. Modern spectroscopic methods and mass spectrometry provide precise elemental analysis. These tools detect isotopes and element signatures, allowing accurate determination of atomic numbers for substances with mixed or unknown composition.



Leave a Comment