How to Know the Difference Between Coplanar and Non-Coplanar
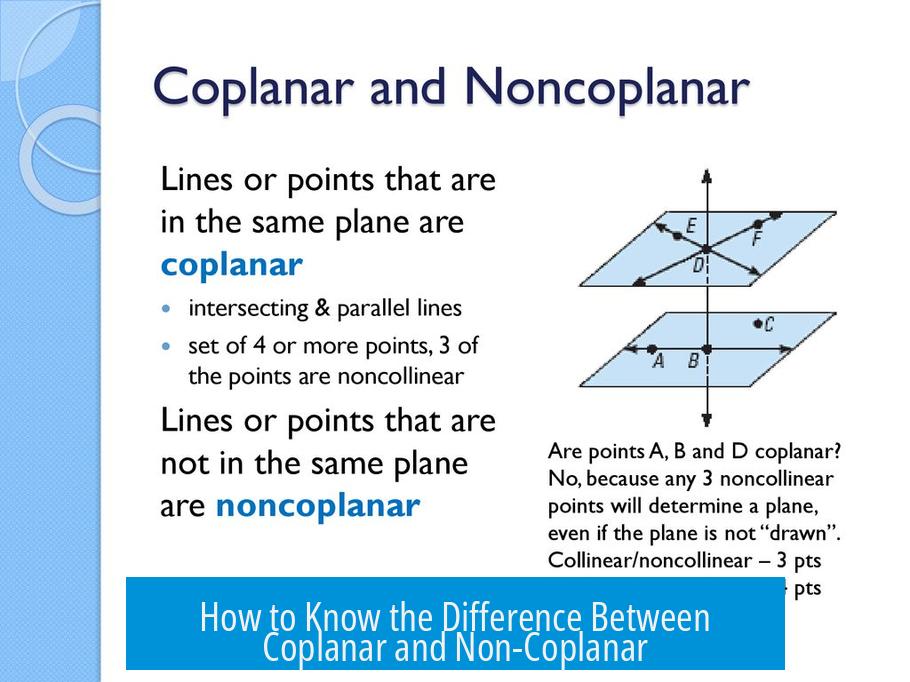 Coplanar and non-coplanar describe the spatial arrangement of groups, especially in molecules. Coplanar groups lie flat on the same plane. Non-coplanar groups do not share the same plane and form an angle.
Coplanar and non-coplanar describe the spatial arrangement of groups, especially in molecules. Coplanar groups lie flat on the same plane. Non-coplanar groups do not share the same plane and form an angle.
Definition of Coplanar
Coplanar means that specific atoms or groups are positioned on the same flat surface. For example, two aryl groups are coplanar if they are parallel and fully aligned in one plane. This arrangement often allows for extended conjugation or overlap of orbitals, which can affect molecular properties such as stability and reactivity.
Definition of Non-Coplanar
Non-coplanar means that groups or atoms do not lie on the same plane. Instead, they form an angle relative to each other. For instance, if two aryl groups rotate so they are tilted or twisted away from perfect alignment, the molecule is non-coplanar. This can reduce orbital overlap and alter chemical behavior.
How to Identify Coplanar vs Non-Coplanar

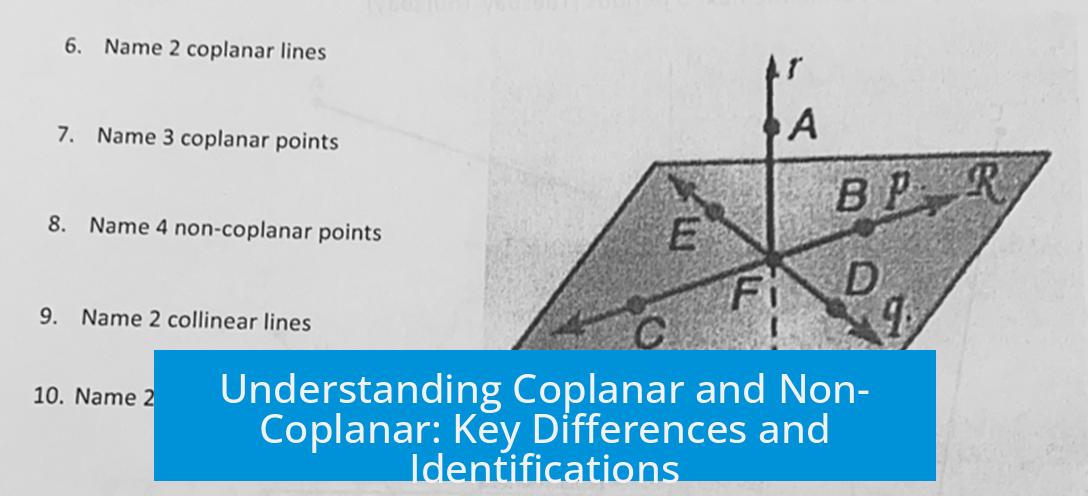
- Visual Inspection: Check if groups lie flat and parallel on one plane (coplanar) or form an angle (non-coplanar).
- Molecular Models: Use ball-and-stick models or software to observe molecular geometry.
- X-ray Crystallography: Provides exact 3D atomic positions to determine planarity.
- Spectroscopic Data: Some spectroscopic features can indicate planarity through conjugation effects.
Significance in Chemistry
Coplanarity affects molecular properties such as electronic conjugation and steric interactions. Planar structures often have more delocalized electrons. Non-coplanar structures may disrupt these effects. Understanding the difference guides prediction of molecule behavior, reactivity, and design of functional compounds.
Summary of Differences
| Aspect | Coplanar | Non-Coplanar |
|---|---|---|
| Position of Groups | On the same flat plane | At an angle, not in one plane |
| Alignment | Parallel and aligned | Tilted or twisted |
| Orbital Overlap | Maximized overlap | Reduced overlap |
| Effect on Properties | Enhanced conjugation and stability | Reduced conjugation, altered reactivity |
- Coplanar molecules have groups aligned in one plane.
- Non-coplanar molecules have groups angled relative to each other.
- Visual and experimental methods help determine coplanarity.
- Planarity impacts electronic and chemical behavior.
How to Know the Difference Between Coplanar and Non-Coplanar
Imagine you’re staring at two flat sheets of paper. If those sheets lie perfectly flat, one on top of the other without bending or tilting, they are coplanar. But twist one slightly, so they no longer align perfectly, and you’ve moved into non-coplanar territory.
When it comes to understanding coplanar vs. non-coplanar in geometry, chemistry, or even physics, this simple idea of “lying on the same plane” versus “not aligning flat” is key.
What Does Coplanar Really Mean?
The term “coplanar” literally breaks down into “co-” meaning together, and “planar” referring to a plane. So, two or more points, lines, or groups that are coplanar share the exact same flat surface.
In chemistry, particularly when talking about aryl groups (which are ring-shaped, flat molecular structures), coplanar means these rings lay flat and align perfectly. Think of two pizza slices laid flat on the table, side by side. Both slices share the same plane, making them coplanar.
“Coplanar means they lay on the same plane, i.e., both aryl groups are flat, parallel, and align on the same plane.”
This precise alignment is important because it often impacts how molecules behave. When groups are coplanar, electrons can flow or interact in predictable ways, affecting everything from molecule stability to color in pigments.
How About Non-Coplanar? What Does That Mean Exactly?
Non-coplanar is a fancy way of saying “not on the same plane.” When objects are non-coplanar, they exist at an angle relative to each other rather than flat and parallel. Imagine slightly tilting one of those pizza slices upwards — now they don’t share the same flat surface anymore.
So, when chemists say two aryl groups are non-coplanar, they mean these molecular rings are tilted or twisted relative to each other.
“Non-coplanar means that the two aryl groups are at an angle with respect to one another i.e., not parallel.”
This misalignment can affect chemical properties dramatically. Non-coplanar arrangements might disrupt electron flow, leading molecules to behave differently. In some drugs or materials, these subtle twists can mean the difference between high reactivity and relative stability.
Why Does It Matter? Benefits of Knowing If Things Are Coplanar or Not
Understanding this difference isn’t just academic. It impacts:
- Chemical reactions: Molecular shape dictates how molecules fit and react. Coplanar molecules often react differently than their non-coplanar cousins.
- Material properties: For materials like polymers or pigments, coplanarity can affect color, flexibility, and strength.
- Drug design: In pharmaceuticals, the shape (including coplanarity) influences drug binding and efficacy.
In short, noticing whether aryl groups or other components are coplanar or non-coplanar helps scientists predict and tailor their behavior.
How to Visually Identify Coplanar vs. Non-Coplanar?
If you’re tackling this on paper, or with a model:
- Look for flat alignment: If components look like they can lie flush on a flat surface with no bending, they’re likely coplanar.
- Check for angles: If parts lean or rotate away from one another, non-coplanar is your answer.
- Use modeling tools: Software or molecular kits help visualize the plane and angles between groups.
Simply put, you want to spot whether these things sit flat together, or if they’re skewed away, breaking the shared flatness.
Personal Tip: Making Sense of This in Real Life
When first learning this concept, I found it helpful to grab a pair of stiff cards or sheets of paper. Holding them together flat versus tilted made the difference crystal clear — a bit like real-life molecular behavior!
In chemistry labs or classrooms, models with flexible bonds let you physically rotate parts and see coplanar vs non-coplanar arrangements in action. This hands-on approach deepens understanding better than just looking at flat pictures.
Comparison Table: Coplanar vs Non-Coplanar at a Glance
| Aspect | Coplanar | Non-Coplanar |
|---|---|---|
| Position | Same flat plane, perfectly aligned | At an angle, not aligned flat |
| Example (Aryl Groups) | Two rings flat and parallel | Two rings twisted or tilted |
| Effect on Electron Movement | Smoother, predictable electron flow | Electron flow is disrupted or less efficient |
| Practical Relevance | Stable molecules, specific reactivity | Altered reactivity, different material properties |
Wrapping It Up: The Bottom Line
So, how do you know the difference between coplanar and non-coplanar? Simply put, coplanar groups lie flat on a common plane, perfectly aligned and parallel. Non-coplanar groups don’t—they tilt or twist, breaking that shared flatness.
Keep this distinction in mind, whether studying molecular geometry or any situation involving flat or angled alignment of parts. This knowledge helps predict properties and interactions that matter in chemistry, materials science, and beyond.
So next time you’re puzzling over molecular shapes, just ask yourself: “Are they flat like a pancake or tilted like a slanted hat?” That’s the quick way to spot coplanar versus non-coplanar.
What does it mean when groups are coplanar?
Coplanar groups lie on the same flat surface. They align so both are parallel and in the same plane.
How can you tell if two groups are non-coplanar?
Two groups are non-coplanar if they form an angle with each other and are not parallel. They don’t lie on the same flat surface.
Why is the alignment of groups important when distinguishing coplanar and non-coplanar?
Alignment shows whether groups share one plane or not. Coplanar groups match flat, while non-coplanar groups tilt at some angle.
Can two aryl groups be partially coplanar?
They are either fully coplanar or at some angle. Partial coplanarity means the groups almost but don’t completely lie on the same plane.
Is being coplanar limited to aryl groups only?
No, coplanarity applies to any atoms or groups lying on the same plane in a molecule or structure.



Leave a Comment