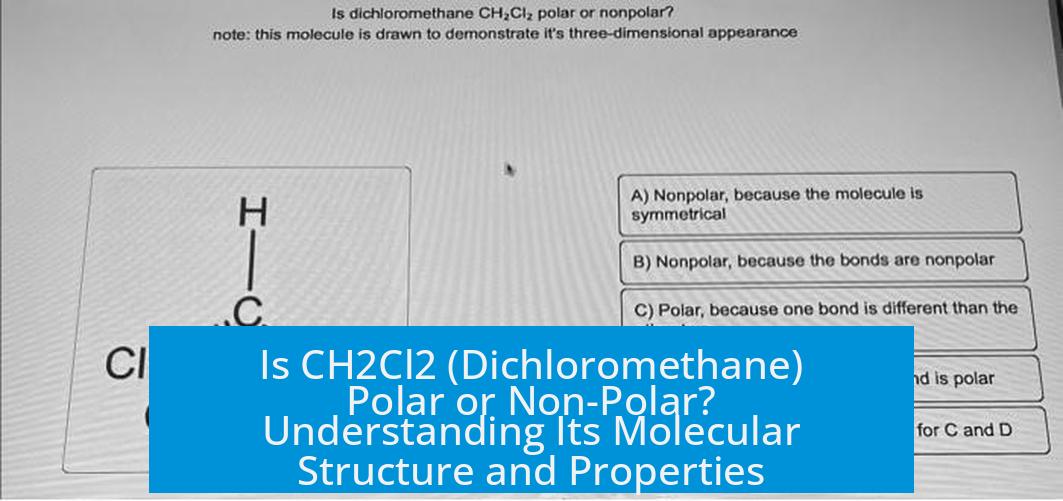
Is CH2Cl2 (Dichloromethane) Polar or Non-Polar?
CH2Cl2 (dichloromethane) is polar. It has a net dipole moment due to its molecular geometry and the difference in electronegativities between its atoms. This polarity places it between nonpolar and strongly polar solvents on the polarity spectrum.
Molecular Structure and Polarity
Dichloromethane has a tetrahedral geometry with two chlorine atoms and two hydrogen atoms bonded to a central carbon atom. The C–Cl bonds are polar because chlorine (electronegativity ~3.16) is more electronegative than carbon (2.55), while the C–H bonds are less polar due to smaller electronegativity differences.
The chlorines are not positioned opposite each other, so their bond dipoles do not cancel. Hydrogen atoms contribute less to the overall dipole. These factors create a net molecular dipole moment. Consequently, CH2Cl2 exhibits weak polarity.
Polarity as a Spectrum
Polarity is not a binary property. Instead, it exists on a spectrum. CH2Cl2 falls in a borderline region between nonpolar and polar solvents. Its dipole moment is stronger than that of hydrocarbons like pentane but weaker than that of highly polar solvents like water or ethyl acetate.
- Hydrogen electronegativity: 2.20
- Carbon electronegativity: 2.55
- Chlorine electronegativity: 3.16
The relative differences lead to moderate polarization of bonds, making the overall molecule weakly polar.
Dielectric Constant as a Polarity Indicator
The dielectric constant approximates solvent polarity. Typical thresholds are:
- <5: Nonpolar solvents
- 5–15: Borderline polar solvents
- >15: Polar solvents
Dichloromethane has a dielectric constant of 9.1, which places it in the borderline polar category. For comparison:
| Solvent | Dielectric Constant | Polarity Category |
|---|---|---|
| Pentane | 1.8 | Nonpolar |
| Ethyl Acetate | 6.0 | Borderline Polar |
| Dichloromethane (CH2Cl2) | 9.1 | Borderline Polar |
| Water | 80 | Polar |
Common Misconceptions and Educational Notes
Some chemistry teaching assistants may inaccurately describe CH2Cl2 as nonpolar. Such misunderstandings often arise from limited experience in synthetic chemistry or an educational focus outside of organic chemistry.
Polarity assessment involves molecular geometry and electron distribution. Dichloromethane’s structure leads to a measurable dipole moment and borderline polar character, which contradicts strict nonpolar classification.
Key Takeaways
- CH2Cl2 is polar with a net dipole moment due to its asymmetrical tetrahedral geometry.
- It is weakly polar, more polar than pentane but less than ethyl acetate or water.
- Dielectric constant (9.1) places CH2Cl2 in the borderline polar category.
- Polarity is a spectrum, not a simple yes/no attribute.
- Educational sources may vary; professional synthetic chemistry contexts affirm CH2Cl2’s polarity.




Leave a Comment