Origin and Reason Behind the Names of Pyrimidines and Purines
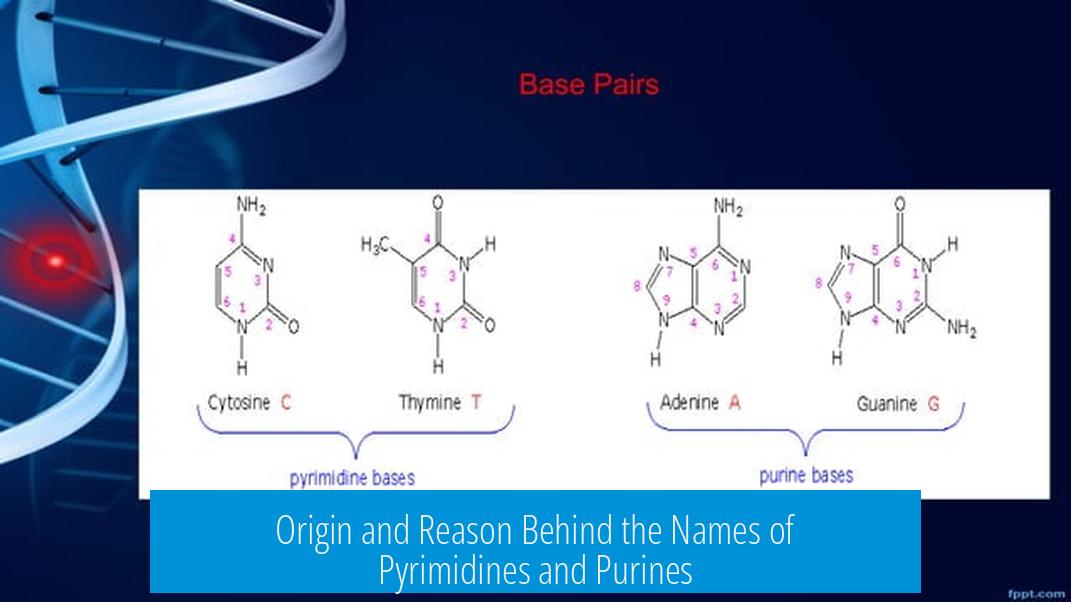
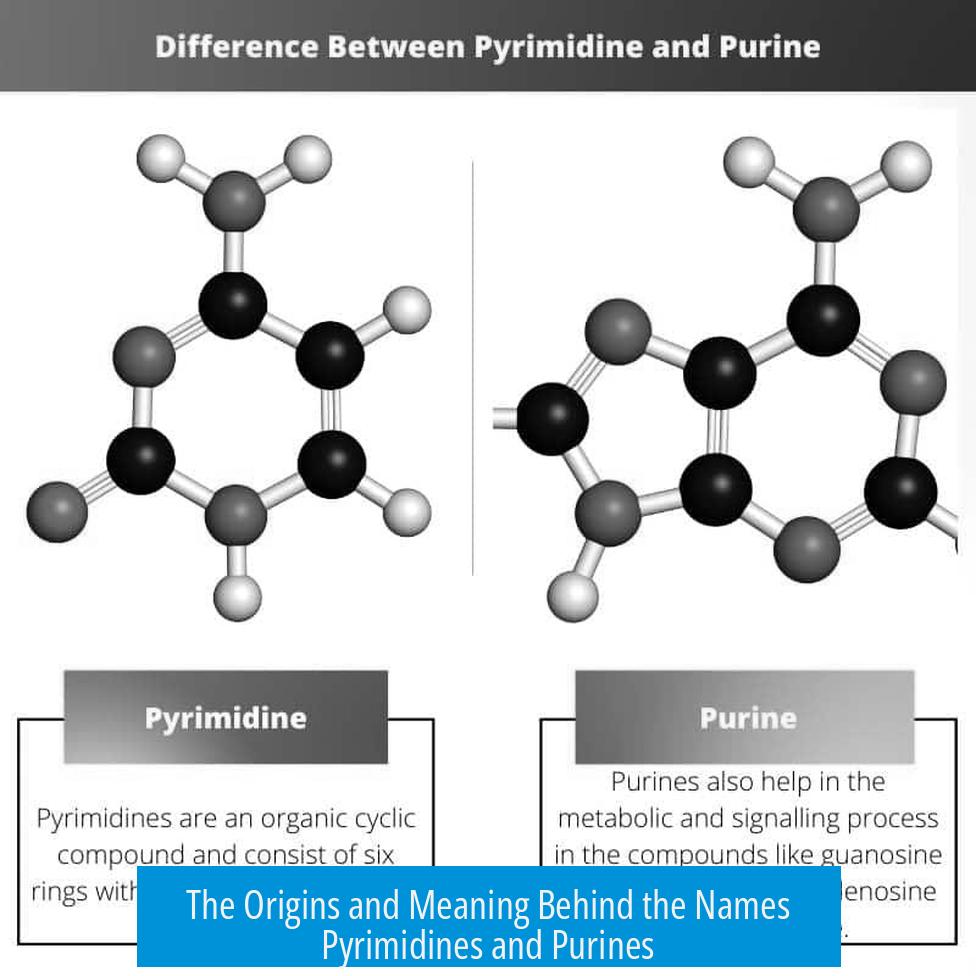
The names pyrimidines and purines originate from their chemical history and structural characteristics. Purines are named after “purum uricum,” Latin for “pure urine,” where they were first isolated. Pyrimidines derive from a combination of chemical terms linked to their structure. The fundamental difference lies in their ring structures: purines contain two fused rings, while pyrimidines have only one.
Historical Background of Purine
The term purine traces back to the Latin phrase purum uricum. This name reflected the early discovery of purine compounds in urine. Early chemists isolated these substances from biological fluids, especially urine samples. The distinct chemical nature of purines led to their recognition as a unique group of organic compounds.
Thus, the nomenclature directly connects to the source of initial isolation rather than chemical structure per se.
Origin of the Pyrimidine Name
The word pyrimidine combines elements that describe its chemical nature and structure. While etymology is less straightforward than purines, it involves several parts:
- pyr-: denotes something flammable or related to fire;
- -idine: signifies nitrogen-containing cyclic compounds, as seen in heterocycles like pyridine;
- mi: believed to derive from amidine, a functional chemical group frequently present in related molecules.
Chemically, pyrimidines can be considered purines without the imidazole ring. Purines have a fused imidazole and pyrimidine ring system, while pyrimidines contain only the six-membered pyrimidine ring. This explains the “taking off the imidazole” idea that leads to pyrimidines.
Structural Differences Define the Classification
The vital distinction between purines and pyrimidines lies in their ring systems:
- Purines contain a dual-ring heterocyclic system: a six-membered pyrimidine ring fused to a five-membered imidazole ring.
- Pyrimidines have only a single six-membered heterocyclic ring.
This structural basis is crucial for biological functionality. Nucleotides incorporate either purine or pyrimidine bases, with base pairing rules depending on the shape and size of these rings.
The single ring in pyrimidines results in smaller bases—cytosine, thymine, and uracil. Purines are inherently larger due to their two rings—adenine and guanine.
The complementary pairing in DNA occurs because pairing a purine with a pyrimidine provides stable hydrogen bonding and appropriate spatial relationships. Two purines paired would be too wide; two pyrimidines too narrow, disrupting DNA stability.
From Chemical Skeletons to DNA Bases
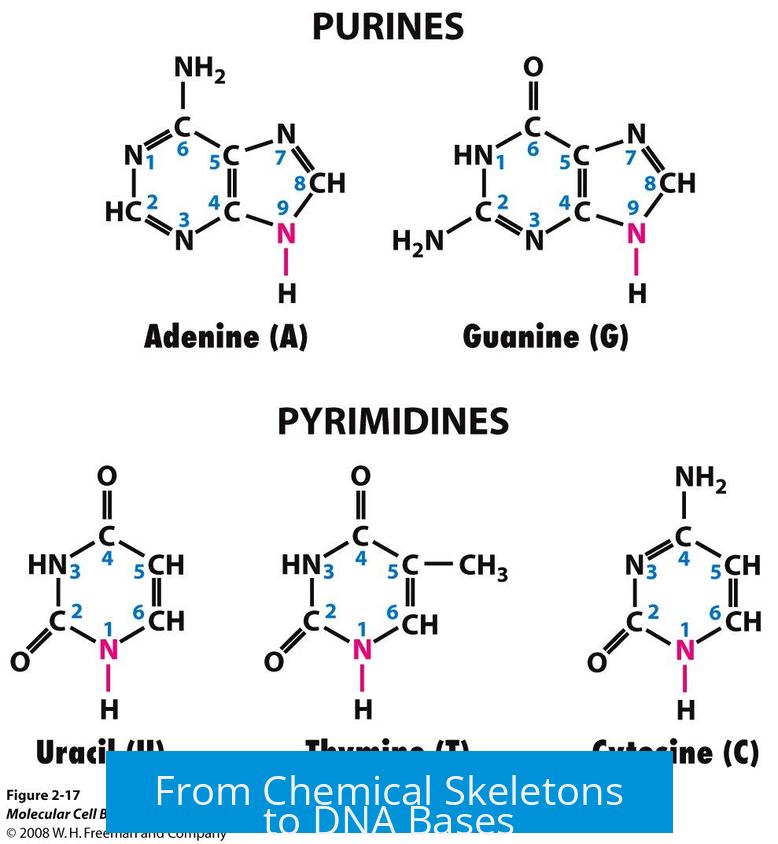
The names purine and pyrimidine originally referred to the heterocyclic skeletons—carbon and nitrogen atoms arranged in rings. As various functional groups like carbonyl and amino groups attach, different bases arise:
| Base Category | Main Heterocyclic System | Examples of Bases |
|---|---|---|
| Purines | Two fused rings (pyrimidine + imidazole) | Adenine (A), Guanine (G) |
| Pyrimidines | Single six-membered ring | Cytosine (C), Thymine (T), Uracil (U) |
Nonetheless, the original heterocyclic backbone dictates classification despite these differences in functional groups.
Mnemonic Devices Versus Scientific Naming
Informal mnemonic devices often simplify how to remember classes of nucleotides:
- CTU (cytosine, thymine, uracil) at the top of a pyramid suggests that these nucleobases belong to pyrimidines. The pyramid analogy recalls the single-ring structure symbolized by the pyramid shape.
- Purines have two rings, which some mnemonic creators associate with purity or pairing, but these stories are not rooted in scientific etymology.
While mnemonics aid memory, these explanations should not be confused with the actual historical or chemical origins of the terms.
Legacy of the Naming
Early chemists named these compounds during the initial exploration of nucleic acid chemistry. The names have persisted due to tradition and their functional relevance. Despite now understanding detailed structures and functions, purine and pyrimidine remain standard nomenclature in biochemical and molecular biology contexts.
Key Takeaways
- Purines derive from the Latin term purum uricum, linked to their discovery in urine.
- Pyrimidines combine chemical prefixes signifying flammability (pyr-) and nitrogenous cyclic structures (-idine), possibly connected to amidines.
- Structural classification is based on ring number: purines have two fused rings, pyrimidines one.
- The chemical skeletons of these compounds define DNA bases and their pairing properties.
- Mnemonic aids exist but are not the origin of these names.
- The naming convention remains from early chemists and is deeply embedded in biochemistry terminology.
Is There a Reason Pyrimidines and Purines Are Called As Such? If So, Why?
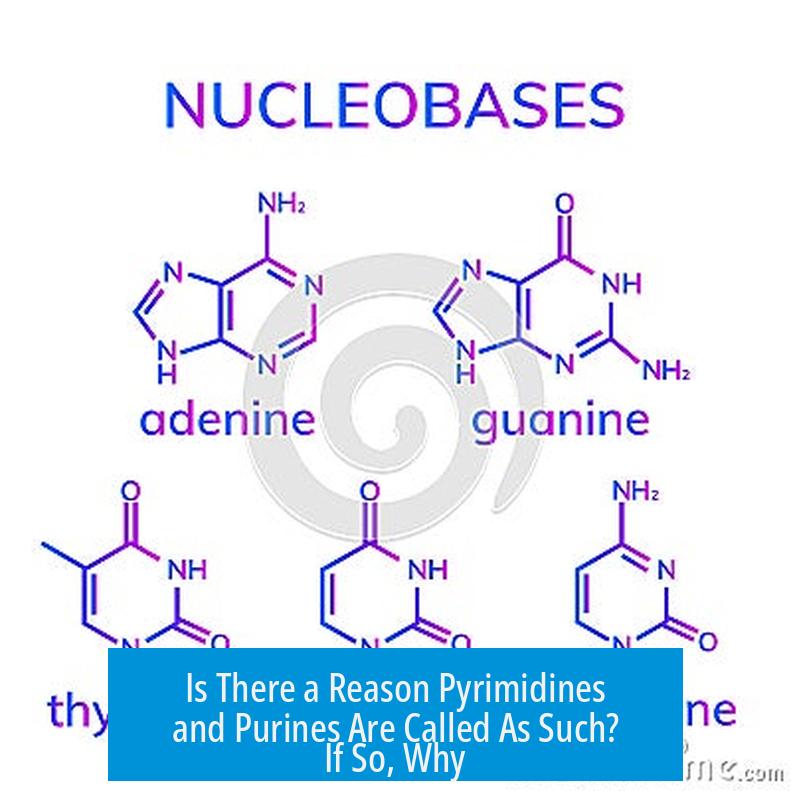
Yes, there’s indeed a reason behind the names pyrimidines and purines, rooted in history, chemistry, and a dash of linguistic creativity.
Pull up a chair; we’re diving into the fascinating story of these nucleotide building blocks—the architects of DNA and RNA. Why those peculiar names? Let’s unfold the tale molecule by molecule.
Purines: Born from Pure Urine (Seriously!)
Ever wonder why purines sound like they belong in a Latin chant? Well, they kind of do. The word “purine” originates from the Latin phrase purum uricum, which translates loosely to “pure urine.” Not because chemists were obsessed with cleanliness in the restroom, but because purine compounds were first isolated from urine back in the pioneering days of chemical research.
This discovery set a precedent that stuck. Purines, bearing this name, carry a certain… flair. They don’t just have any structure; they sport a two-ring fused setup, a heterocyclic duo—a pyrimidine ring fused with an imidazole ring. This duo ring structure is crucial because it influences how the molecule pairs up and interacts, preventing weird bonding scenarios.
Pyrimidines: A Single Ring with a Fiery Name
Pyrimidines might sound like a fancy pyramid dessert, but their name has a different origin. Chemically, pyrimidines are like stripped-down purines—literally purines minus the imidazole ring. They contain just one nitrogen-containing heterocyclic ring.
The name itself is an intriguing mix:
- pyr-: derived from the Greek word for fire or flammable material.
- -idine: indicates a nitrogen-containing cyclic compound.
- mi-: believed to come from “amidine,” a specific chemical group present in the molecule.
So, “pyrimidine” effectively hints at a nitrogenous ring that’s somehow *flammable*—though don’t try lighting your DNA on fire just yet!
The Structural Reason These Names Matter
Why fuss over names? Because the structure defines how these molecules behave inside living cells. Purines, with their two rings, and pyrimidines, sporting a single ring, pair up in DNA and RNA to create the famous double helix and other complex structures.
This ring difference is a molecular rulebook. If two pyrimidines tried to pair up, the DNA strand would be too short and squished. Two purines? Too bulky and pushing apart the hydrogen bonds that hold DNA together like molecular glue.
The careful balance between these rings ensures DNA’s stability and function. The names are essentially a label on the blueprints of life’s building blocks.
Mnemonic Fun: Remembering Rings with Humor
If all these chemical origins sound too dry, here’s a fun way chem students remember the difference: picture a CTU—a Counter Terrorist Unit—atop a pyramid. CTU stands for cytosine, thymine, and uracil, all pyrimidines. The pyramid evokes “pyrimidine,” reminding learners these bases have a single ring.
And for purines: there’s a silly saying that people are “pure” when they marry, so purines have two rings, like a wedding ring set. Not scientifically accurate, but memorable! Sometimes humor best bridges the gap between chaos and clarity.
Why These Names Stuck Around
Chemists of the past named these molecules based on sources, structure, and chemistry knowledge of their times. The names purine and pyrimidine sound archaic but are deeply rooted in scientific history and tradition.
Even though newer naming conventions could be clearer, these terms persist because they’re entrenched in textbooks, research, and teaching worldwide—kind of like an old inside joke among biochemists.
What’s the Big Picture?
Understanding why purines and pyrimidines are called what they are is more than trivia. It reveals the story behind the science — the intersection of history, chemical structure, and biological function.
- Purines emerge from their first isolation from urine, recognized by their fused two-ring structure.
- Pyrimidines have a single ring, with names inspired by notions of flammability and nitrogen rings.
- The structural difference determines how they pair and stabilize DNA.
- Names stuck due to historical convention, not just random naming.
- Mnemonics and quirky explanations help memorization but aren’t the etymological source.
So next time someone asks, “Why are those DNA bases called purines and pyrimidines?” you’ll have the perfect story—a fascinating blend of urine, fire, rings, and old chemistry lore.
Practical Tips for Students and Curious Minds
Want to remember these better?
- Use the CTU pyramid image. It’s surprisingly sticky in your memory.
- Recall “purum uricum” for purines to associate their origin.
- Focus on the ring structure difference; it explains their pairing logic.
- When studying DNA or RNA, visualize how the two-ring and single-ring shapes fit together.
Embrace the weird origins—they make science human and relatable.
Conclusion
Yes, there’s a real reason behind the names purines and pyrimidines. These names reflect both their chemical structures and their discovery history, grounded in ancient nomenclature that has, remarkably, stood the test of time. Their rings aren’t just chemical circles; they’re symbols of the molecular dance life performs daily inside every cell.



Leave a Comment