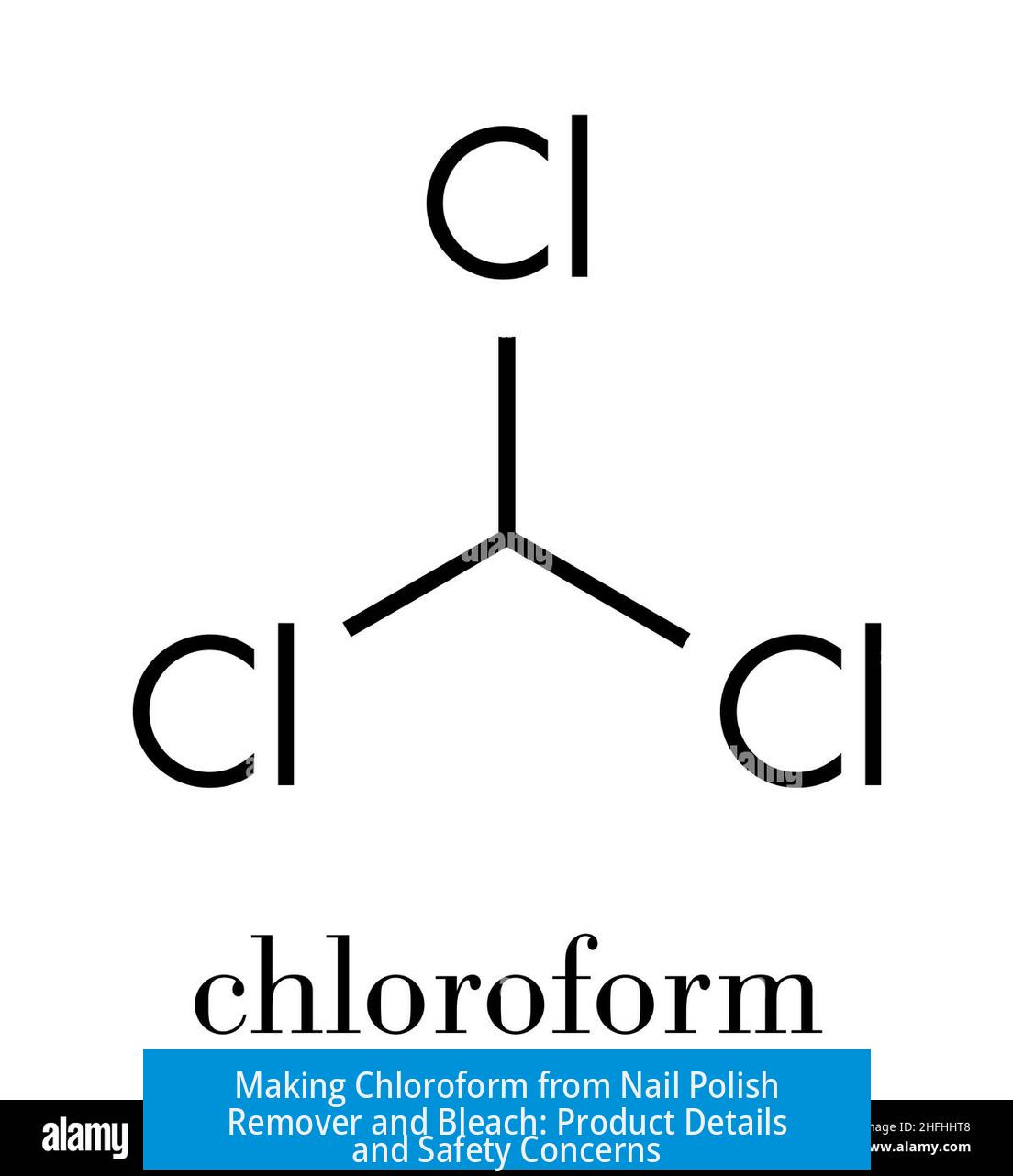
Making Chloroform from Nail Polish Remover and Bleach: Analysis and Cautions
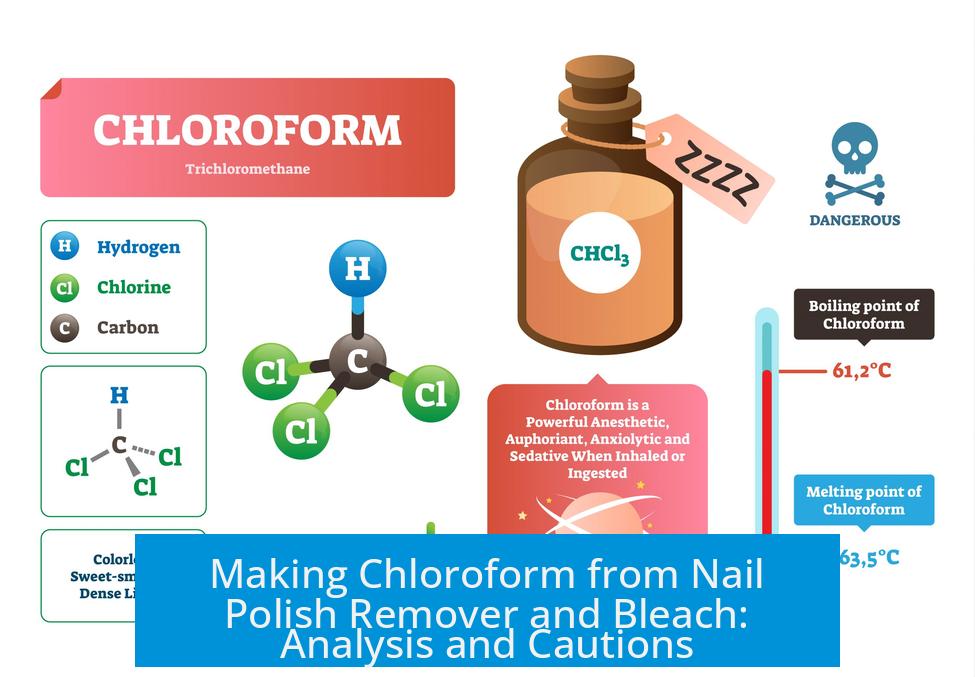
Chloroform can be produced through the reaction of acetone, a primary component of nail polish remover, with bleach. The resulting product is typically clear; any coloration suggests impurities or side reactions. This synthesis involves serious safety risks, including the possible formation of explosive compounds. Proper purification and storage are essential.
Chemical Reaction Overview
The preparation involves mixing acetone (from nail polish remover) with sodium hypochlorite (bleach). This reaction under alkaline conditions yields chloroform (CHCl3) and other byproducts. The equation can be summarized as:
3 NaOCl + CH3COCH3 → CHCl3 + 2 NaOH + CH3COONa
However, the reaction requires controlled conditions and purification steps to obtain pure chloroform. The appearance of any coloration usually indicates incomplete reaction, residual reactants, or formation of side products.
Color and Purity of Final Product
Pure chloroform is a clear, colorless liquid. If the final product appears colored, it may contain impurities such as chlorinated acetone derivatives or decomposition products. These contaminants may affect both the chemical behavior and toxicity of the sample.
Safety and Health Risks
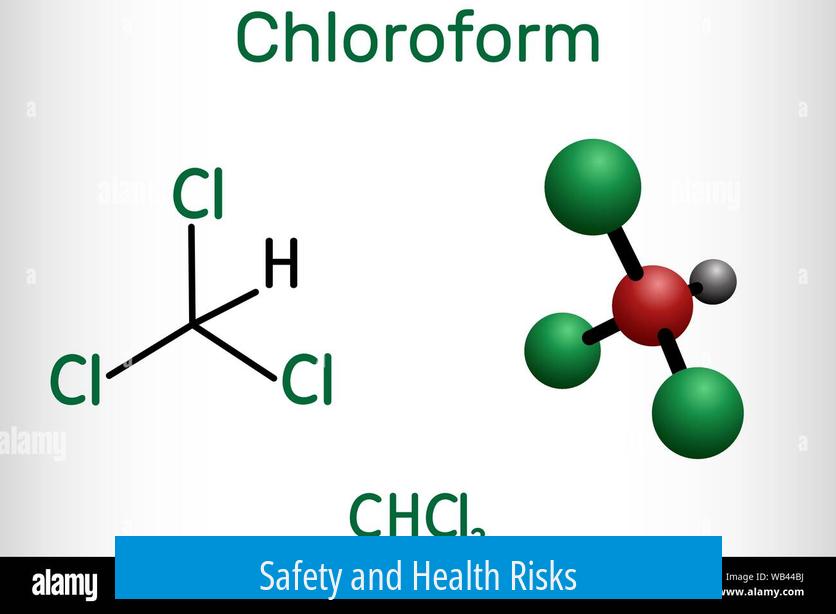
- Mixing acetone and bleach can generate hazardous substances aside from chloroform, including trichloromethyl radicals and potentially explosive triacetone triperoxide (TATP).
- TATP is a powerful explosive linked to dangerous incidents, cautioning against unsupervised synthesis.
- Proper purification, such as drying and distillation, is critical to remove residual bleach, acids, and byproducts that could cause chemical burns or toxic exposure.
- Storage must be in airtight, UV-protected containers away from heat sources to avoid degradation or accidental release of toxic vapors.
Use and Testing Considerations
- Chloroform is an industrial solvent and was historically used as an anesthetic; it is now largely restricted due to its toxicity and carcinogenicity.
- Testing for purity requires analytical techniques like gas chromatography or NMR spectroscopy.
- Any contact, ingestion, or inhalation poses significant risks and should be avoided outside professional settings.
Reference to Demonstration Video
A video detailing the process is available here: Synthesis of Chloroform from Nail Polish Remover and Bleach. It illustrates the practical steps but does not replace safety training or proper chemical handling protocols.
Key Takeaways
- Chloroform can be formed by reacting acetone (nail polish remover) with bleach under controlled conditions.
- Pure chloroform is colorless; color suggests impurities or incomplete reaction.
- The synthesis carries serious risks, including explosive byproduct formation and toxic exposure.
- Proper drying, distillation, and secure storage are essential for safety and product quality.
- Chloroform is toxic; use and testing should only occur with proper knowledge, equipment, and precautions.
Is the color of the chloroform normal after making it this way?
Pure chloroform is usually clear. The coloring may come from impurities or side reactions during the process. The final look can vary based on materials used and reaction conditions.
What safety steps should be taken after making chloroform with these chemicals?
Drying and distilling the product are important to purify it. Proper storage is also crucial to avoid degradation or hazards. Skipping these steps can increase risks.
Can mixing nail polish remover and bleach create dangerous compounds?
Yes. Acetone in nail polish remover mixed with bleach can sometimes form TATP, a strong explosive. This risk makes the process very unsafe without proper control.
What are common uses for homemade chloroform?
Some ask about practical uses, but homemade chloroform usually lacks purity for safe applications. Experimental curiosity often drives attempts rather than practical use.
Has the chloroform made through this method been tested for effectiveness or purity?
Testing is rarely shown publicly. Purity often varies, and untested products may be unreliable or unsafe. Verification requires professional equipment.



Leave a Comment