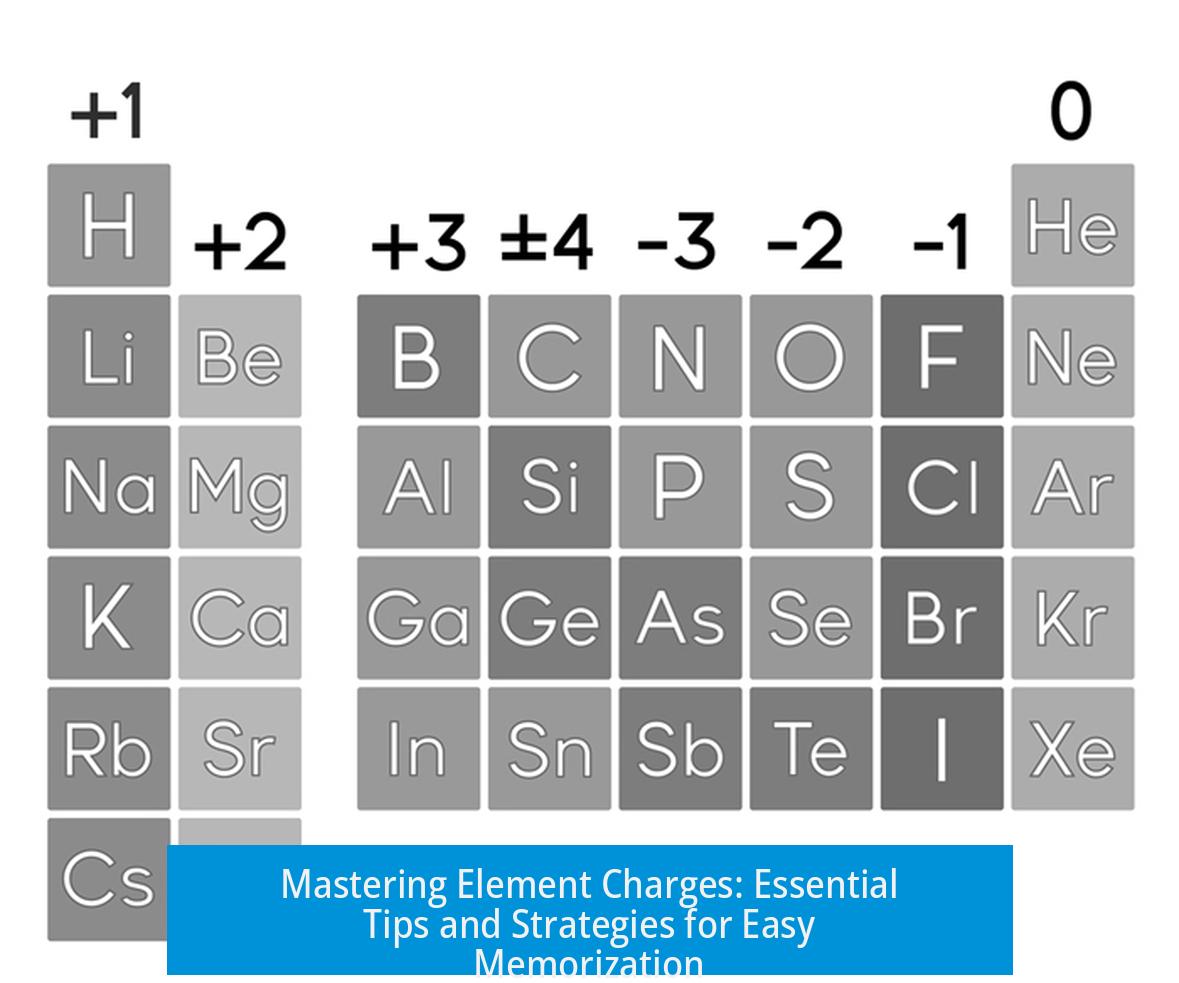
Memorizing the Charges of Elements?

Memorizing the charges of all elements is generally unnecessary; instead, focus on common oxidation states and specific groups. Most chemistry courses and contexts require familiarity only with certain key elements and group trends rather than memorizing every possible charge.
Which Elements to Memorize?
Students often need to remember charges for a select set of transition metals such as manganese (Mn), chromium (Cr), cobalt (Co), iron (Fe), nickel (Ni), copper (Cu), cadmium (Cd), zinc (Zn), silver (Ag), gold (Au), mercury (Hg), platinum (Pt), tin (Sn), and lead (Pb). These commonly appear in problem sets and quizzes.
- Fe, Co, Ni mostly have +2 and +3 oxidation states.
- Cu has +1 and +2 states, with +1 linked to pennies for memory aid.
- Pb and Sn typically show +2 and +4 charges.
- Cr can have +2, +3, and +6 states, making it somewhat irregular.
- Au usually has +1 and +3 states.
Memorization Tips
Mnemonic devices can aid retention. For example, linking nickel (Ni) to the value of 5 cents helps recall +2 and +3 states (2+3=5). Visual aids like index cards and repeated review assist memorization. Studying with peers enhances retention through discussion.
Conceptual vs. Memorization Approach
Understanding underlying concepts, such as hybridization, Hund’s rule, and electron configurations, complements memorization. This approach helps identify why certain elements favor specific charges rather than rote learning all possibilities.
Groups 1, 2, 14–18 usually have predictable charges, derived from valence electron counts, so memorizing these is beneficial. Transition metals show variable oxidation states and often require contextual inference rather than strict memorization.
Context-Driven Learning
Recognize the context of questions or problems. For general chemistry, experts recommend focusing on group trends and charges in compounds rather than isolated elements. Transition metals’ charges are often inferred by the compound’s composition rather than recalled outright.
Summary of Key Points
- Memorize charges of common transition metals and group elements instead of all elements.
- Use mnemonics for elements like Fe, Ni, Cu, Pb, Sn, and Cr to simplify recall.
- Leverage conceptual understanding of electron configuration and chemical behavior.
- Utilize study aids such as flashcards and group study for effective memorization.
- Focus on group trends and context when determining element charges in compounds.
Memorizing the Charges of Elements? 🙁 Let’s Make It Easier
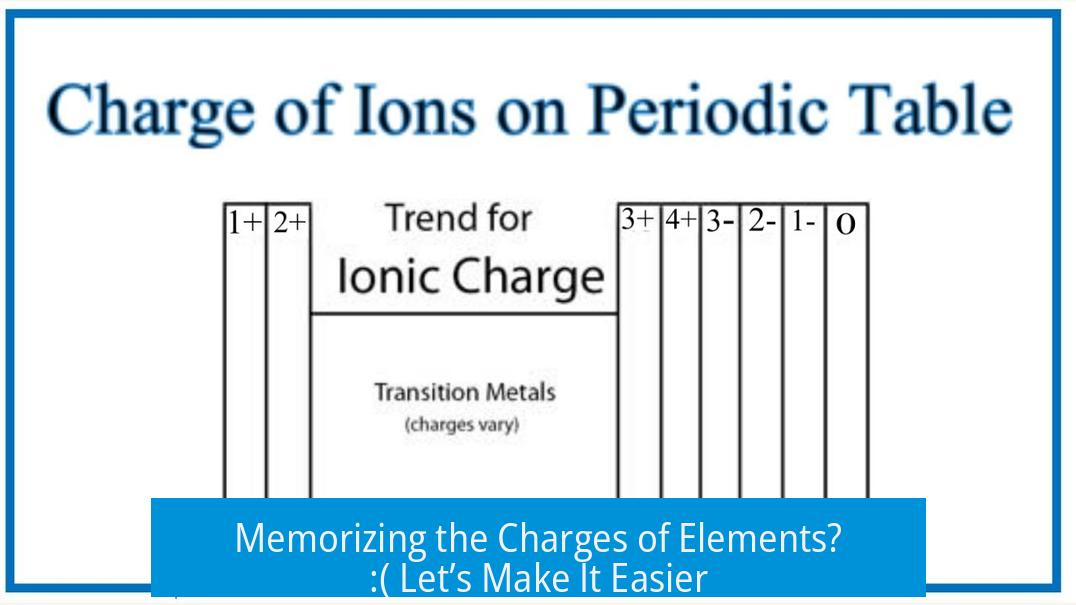
Is it really necessary to memorize the charges of every element? The short answer: No, at least not all of them. Many chemistry students face this dreaded task with a sigh. One senior in university shares that despite nearing the end of their chemistry journey, they’ve never had to memorize oxidation states. Intriguing, right? Maybe it’s a regional quirk or teaching style difference. Some regions demand strict memorization, others lean on conceptual understanding.
This post digs into those mysteries, sharing tips, mnemonics, and strategies to make memorizing the charges of elements less of a nightmare.
Do You Really Need to Memorize All Charges?
Here’s a secret: you probably don’t. Certain groups have charges that follow clear trends that are easy to predict. For example, alkali metals (group 1) almost always have a +1 charge, alkaline earth metals (group 2) +2, and nonmetals in groups 15, 16, and 17 often take on predictable negative charges. Instead of rote memorization, understanding periodic trends helps. That’s chemistry Sherlock Holmes style!
Transition metals, however, are the wildcards. They don’t follow simple charge rules. A chemistry teacher recalls their own tough days memorizing various transition metal charges well before the International Baccalaureate (IB) made it a requirement. Despite struggles, knowing these charges has practical use later. However, it’s not necessary to know the full periodic table’s oxidation states cold—just the key troublemakers.
Which Elements Should You Focus On?
Based on real student experience, focusing on some key elements pays off. One professor had students memorize the charges of these:
- Manganese (Mn)
- Chromium (Cr)
- Cobalt (Co)
- Iron (Fe)
- Nickel (Ni)
- Copper (Cu)
- Cadmium (Cd)
- Zinc (Zn)
- Silver (Ag)
- Gold (Au)
- Mercury (Hg)
- Platinum (Pt)
- Tin (Sn)
- Lead (Pb)
This list covers most common transition metals encountered in homework and quizzes. While impressive, it’s not the entire periodic table, and that’s good news for your brain cells.
Mnemonics to the Rescue
Here’s where memory tricks shine. They turn dry chemistry tables into stories that stick:
- Fe, Co, Ni: These neighbors all boast +2 and +3 charges. Remember nickel as “5 cents,” so 2+3=5. Simple math meets chemistry.
- Cu: Has both +1 and +2. Think pennies for +1—everyone remembers pennies. Just don’t forget copper’s fancy side, +2.
- Au: Gold is special with +1 and +3 charges. Like a king of elements, it walks its own path.
- Pb and Sn: These are always homework troublemakers with +2 and +4 charges. Keep them paired in your mind.
- Cr: Chromium is the drama queen with +2, +3, and +6. Crazy and undecided, just like the mnemonic says.
Mnemonics are your BFFs here; they help turn abstract numbers into memorable mental images.
Strategies That Actually Work
Not a fan of memorization? You’re not alone. One senior student admits that even they struggle with memory but can recall these charges fast, thanks to these:
- Use index cards. Flip through them during downtime. Consistency beats cramming.
- Find a study buddy. Explaining concepts aloud cements memory better than solo mugging.
- Focus on understanding trends instead of raw numbers, especially for groups with predictable charges.
Patience and repetition win the day.
Understanding Beats Memorizing — Sometimes
Knowing basic chemistry principles can bypass the need to memorize every detail. For instance, orbital hybridization, Hund’s rule, and high-spin vs. low-spin states give clues to oxidation states of transition metals. Not every course requires this detail, though.
You might ask yourself: does the exam need the full set of charges, or just the common ones? Typically, knowing groups 1, 2, 14 to 18 elements’ charges is enough. Use logic for transition metals depending on the chemical context.
Why Are Transition Metals So Tricky?

The transition metals drop the chemistry textbook logic bomb: their charges vary with the compound and environment. Iron can be +2 or +3, chromium can swing among +2, +3, and +6. This variability adds a layer of complexity. Instead of pure memorization, experience helps.
Years of exposure to these elements in chemical reactions naturally solidify their common charges. Those who don’t memorize may still pick them up by working through problems.
Context Is King
One of the best tips? Keep your context clear. If you’re asked to identify charges when transition metals form compounds, the situation usually limits likely charges. For general chemistry, being familiar with the standard charges for main groups is essential. For tricky transition metals, using clues from the compound’s other elements helps deduce the charge, instead of mindless rote.
Final Thoughts: It’s Not Just Memorization, It’s Strategy
Memorizing charges of elements isn’t a one-size-fits-all challenge. It’s part memory game, part logic puzzle. Focus on essential elements, use mnemonics, and reinforce by practice. Don’t stress over memorizing the entire periodic table’s charges unless your course requires it.
So next time you see a charge question, instead of groaning, ask: “What’s the teaching style here? What’s the context? Can I make a mnemonic to tame this beast?” You’ll be surprised how manageable this chemistry chore becomes.
Remember, even chemistry seniors find this tricky—if they can master it, so can you!
Q1: Which elements’ charges do I really need to memorize?
The most commonly memorized elements include Mn, Cr, Co, Fe, Ni, Cu, Cd, Zn, Ag, Au, Hg, Pt, Sn, and Pb. Focus on these since homework and quizzes often ask about them.
Q2: Are there easy ways to remember charges for tricky transition metals?
Yes. For example, Fe, Co, and Ni have 2+ and 3+ charges; remember nickel “adds up” to 5 (2+3=5). Cu is 1+ and 2+, like pennies, and Cr is 2+, 3+, and 6+, because it “can’t decide.”
Q3: Is memorizing all oxidation states necessary?
Not always. Learning common group charges helps most. Transition metals vary a lot, so understanding trends and context can reduce the need for full memorization.
Q4: What strategies help with memorizing element charges?
Use index cards and review often. Studying with a partner can also help. Regular practice helps recall these charges faster over time.
Q5: How does understanding chemistry concepts reduce memorization?
Knowing orbital hybridization, electron placement, and spin states helps predict charges. This approach is better than rote memory and aids problem-solving.




Leave a Comment