NMR Terminology: Understanding Upfield and Downfield

In nuclear magnetic resonance (NMR), “upfield” refers to signals at lower ppm values where nuclei are more shielded by electrons, experiencing a weaker magnetic field. Conversely, “downfield” denotes signals at higher ppm values where nuclei are less shielded and require a stronger magnetic field to resonate.
Chemical Shift and Electron Shielding
Chemical shift in NMR arises from how electrons around a nucleus shield it from the external magnetic field. Nuclei with high electron density feel a reduced effective magnetic field, shifting their signals to lower ppm values. These locations in the spectrum are called upfield.
In contrast, nuclei with fewer surrounding electrons undergo less shielding, experiencing almost the full external field strength. Their signals appear at higher ppm values, labeled downfield.
Why the Terms Upfield and Downfield?
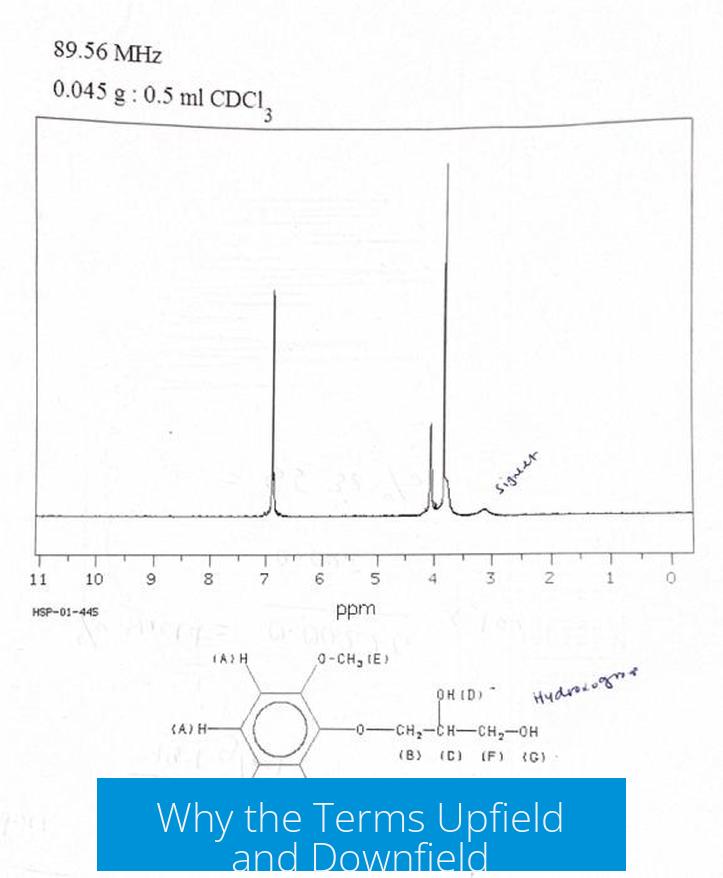
The terms come from original NMR methods before Fourier transform was common. Early instruments swept the magnetic field strength while keeping radiofrequency constant. “Downfield” meant resonance occurred at a higher field strength, and “upfield” meant resonance at a lower field.
After Fourier transform adoption, the practice reversed: the magnetic field stays constant while radiofrequency varies. Still, the old terminology persists.
The Reversed ppm Scale
The NMR horizontal axis shows chemical shift in ppm, describing resonant frequencies relative to a standard reference, usually tetramethylsilane (TMS). The axis commonly runs right to left, with high ppm (downfield) signals on the left and low ppm (upfield) on the right.
This reversed scale reflects the historical method where magnetic field strength was the variable. Signals requiring a stronger field appear on the left side of the spectrum, making them downfield.
Conceptualizing Magnetic Fields in NMR
- The external magnetic field is constant during data acquisition today.
- Terminology assumes a mindset from earlier instruments with variable field strength.
- Downfield signals indicate nuclei resonating at a higher effective magnetic field than the reference.
Key Points to Remember
- Upfield means lower ppm, more electron shielding, weaker effective magnetic field.
- Downfield means higher ppm, less shielding, stronger effective magnetic field.
- The ppm scale is reversed due to historical magnetic field sweeping methods.
- TMS is the common reference point at 0 ppm.
- Modern instruments keep magnetic field constant and vary radiofrequency.
NMR Terminology: Upfield and Downfield
Ever stared at an NMR spectrum and wondered what “upfield” and “downfield” actually mean? Here’s the scoop: In NMR, signals that appear at lower ppm values are termed upfield and those at higher ppm values are downfield. This corresponds to how shielded the atoms are from the magnetic field. Why? Because atoms with more electron clouds around them are more shielded—they feel less magnetic field influence, so their signals show up “upfield” at lower ppm.
Let’s break this down with a pinch of humor and a dash of science.
Imagine you have a proton wearing a tiny shield made of electrons. The thicker this shield, the less force it feels from the big external magnet in the NMR machine. The result? It requires less energy to “excite” or “flip” that proton’s spin, which means it resonates at a lower ppm value. That’s the essence of being upfield.
How Electron Shielding Rules the Chemical Shift Dance
Shielding is the game-changer here. The more electrons near a nucleus, the more “protected” it feels and the closer its signal huddles towards lower ppm values on the spectrum. These signals are “upfield.” In contrast, nuclei with fewer electron clouds around have less shielding. They feel the magnetic field more intensely and show signals at higher ppm—labeled as “downfield.” Think of upfield protons as introverts hiding behind a curtain, while downfield protons are extroverts standing in bright spotlight.
Why Do We Call It Upfield and Downfield Anyway?
The terminology comes from a classic conceptual model. Originally, NMR instruments kept the radiofrequency constant and varied the magnetic field strength to detect resonance. Protons that were less shielded required a stronger magnetic field to flip, so their resonance signals appeared “downfield”—at higher magnetic field strengths. Conversely, shielded protons needed a weaker magnetic field, hence showing “upfield” signals.
This historical setup perfectly explains why the x-axis (the chemical shift or ppm scale) on modern NMR spectra is reversed compared to what you might expect. We plot from high ppm on the left (downfield) to low ppm on the right (upfield), echoing those old varying magnetic field methods.
Keeping the External Magnetic Field Constant: A Mental Shift
Using modern Fourier transform NMR, the external field stays constant, and the radiofrequency is swept instead. But the terminology sticks. It’s like calling a parking space by its old landmark long after the building was replaced.
In practical terms, “downfield” signals come from nuclei that resonate at a smaller effective magnetic field strength compared to the standard reference compound—usually tetramethylsilane (TMS). The TMS peak is shiny and often taken as zero ppm. Protons appearing downfield resonate at magnetic fields lower than TMS under constant external field and fixed radiofrequency conditions. It’s a little paradox, but just a quirk of the system and terminology combined.
Wrapping It Up: What Does This Mean for Your NMR Spectra?
When you analyze an NMR spectrum, focus first on the ppm scale. Peaks to the left (higher ppm) indicate protons in less electron-rich environments—think acidic hydrogens or aromatic rings—these are your downfield stars. Peaks to the right (lower ppm) come from shielded protons—like those nestled in methyl groups or alkyl chains—your upfield crowd.
In practice, an aromatic proton usually shows up around 7-8 ppm—definitely downfield compared to a methyl proton near 0.9 ppm, which is upfield. This can help identify functional groups or molecular environments quickly.
In a Nutshell: Why Should You Care?
- Understanding upfield vs. downfield gives you a shortcut to the electronic environment around nuclei.
- NMR spectra become more than squiggles—they turn into stories about molecular structure and behavior.
- This awareness helps you predict shifts and understand chemical reactions, bonding, and molecular architecture.
- It clears up confusion over the reversed ppm scale, making your spectrum readings less of a guessing game.
Curious for More?
Did you know that shielding effects vary so much that protons near electronegative atoms (like oxygen or nitrogen) shift significantly downfield? Meanwhile, protons in shielded environments, like in electron-rich aromatic rings, can flip the expected positions. This quirky behavior keeps spectroscopists on their toes! Next time you see an unusual peak placement, remember, it’s all tied to electron clouds—and your trusty “upfield” and “downfield” compass.
If you are just diving into NMR, try mapping different protons in a familiar molecule with this upfield/downfield concept. It makes the spectra almost like a treasure map, guiding you to hidden molecular secrets.
Bottom line? Armed with the right terminology and context, your NMR interpretation gains clarity and confidence. So next time you encounter “upfield” or “downfield,” smile knowingly—you’re speaking fluent molecular magnetic!
What does “upfield” mean in NMR spectroscopy?
Upfield signals occur at lower ppm values. These protons are more shielded by electrons and experience a weaker effective magnetic field.
Why is the NMR ppm scale reversed on the x-axis?
The scale is reversed because historically the magnetic field was scanned while radiofrequency stayed constant. Upfield and downfield relate to changes in magnetic field strength.
How does electron shielding affect chemical shift in NMR?
More electrons around a nucleus shield it from the magnetic field. This shifts its signal upfield, resulting in a lower ppm value.
Why are terms like upfield and downfield still used if the external magnetic field is constant?
These terms reflect an older way of thinking when magnetic field strength was varied. Now, they describe resonance positions relative to TMS under constant external field and frequency.
What does “downfield” signify in NMR readouts?
Downfield signals appear at higher ppm values. These protons resonate at a higher effective magnetic field because they are less shielded.



Leave a Comment