Ortho, Para and Meta Directors in Aromatic Substitution
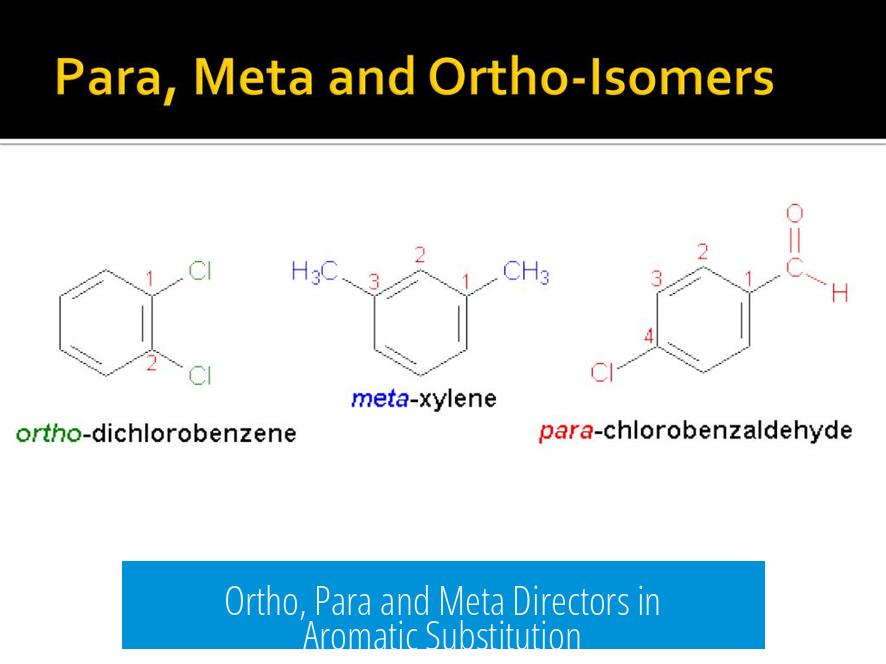
Ortho, para and meta directors refer to how substituents on a benzene ring influence the position of new substituents in electrophilic aromatic substitution reactions.
Directing Effects: A Mixed Trend
Substituents do not direct substitutions purely to one position. Instead, each group favors either the ortho, para, or meta position more strongly, but always produces a mixture. Even strong directors produce some products in minor positions.
- Ortho and para directing groups favor addition adjacent (ortho) or opposite (para) to themselves.
- Meta directing groups favor substitution at the meta position, which is one carbon removed from ortho.
Ortho/Para Directors Example
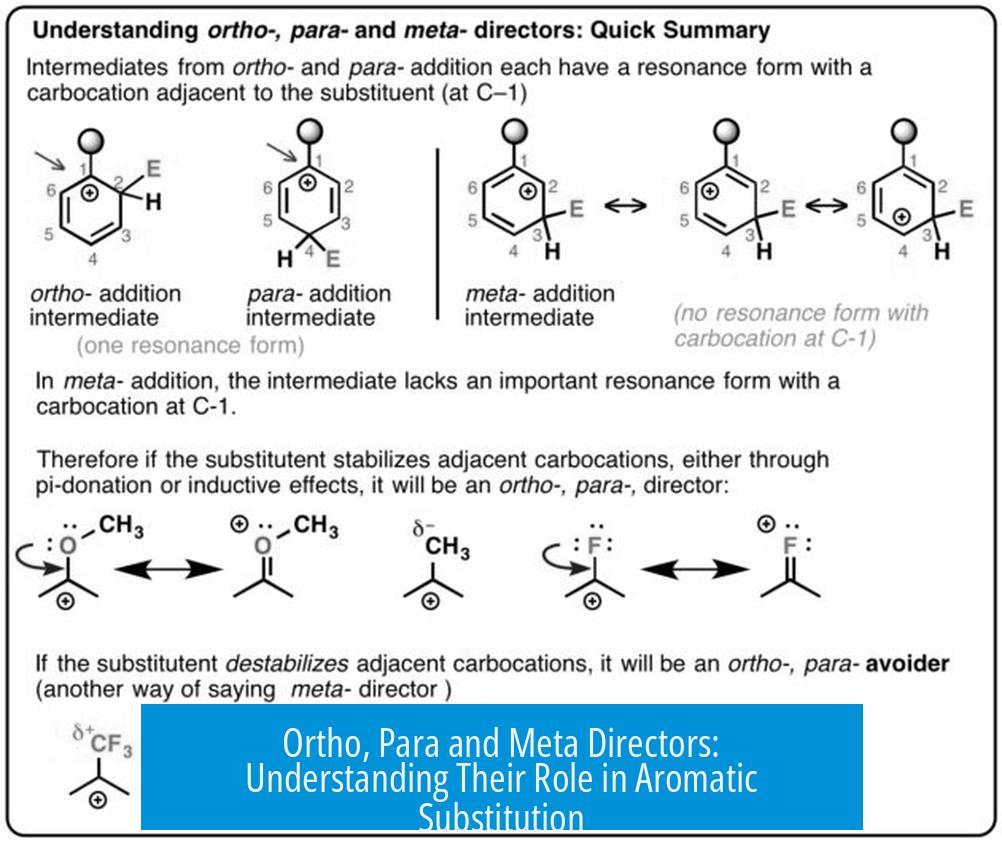
Electron-donating groups typically act as ortho/para directors. For example, methoxy (-OCH3) groups direct new substituents mostly to ortho and para positions.
Still, methoxy substituents produce a small percentage of meta products. This shows directing effects are not absolute but are dominant preferences.
Meta Directors Example
Electron-withdrawing groups like trifluoromethyl (-CF3) are meta directors. Substitution occurs mostly at the meta position relative to the substituent.
However, meta directors still yield minor amounts (up to 10%) of ortho and para products.
Weak Directors and Regioselectivity
Groups such as CH2X or CHX2 possess weak directing abilities. Their regioselectivity is low and depends on the halogen X.
Results may slightly favor ortho/para or meta positions, but often yield non-selective mixtures.
Practical Strategies for Good Regioselectivity
For precise functionalization, it is common to introduce strongly directing groups first, then add substituents like halogens later.
For example, synthesizing 3-bromomethylnitrobenzene can involve:
- Nitration of benzaldehyde, producing primarily meta-nitrobenzaldehyde
- Reduction of the aldehyde to alcohol using sodium borohydride
- Substitution of alcohol with bromine using phosphorus tribromide (PBr3)
This sequence leverages the directing effects to control substitution positions.
Key Takeaways
- Directing effects always produce a mixture but favor either ortho/para or meta substitution.
- Methoxy groups are strong ortho/para directors but allow minor meta substitution.
- CF3 is a meta director but gives some ortho/para products.
- Weak directors have low regioselectivity and inconsistent directing tendencies.
- Synthetic planning often introduces groups in steps to maximize regioselectivity.
What determines if a substituent is ortho/para or meta directing?
No substituent is completely ortho/para or meta directing. All give a mix, but favor more of one type, depending on their nature.
Can strong ortho/para directors produce meta-substituted products?
Yes. For example, methoxy is a strong ortho/para director but still forms a small amount of meta products.
Why do weak directors show poor regioselectivity?
Groups like CH2X or CHX2 are weak directors and may slightly favor ortho/para or meta, but overall regioselectivity is low.
How can I improve regioselectivity in substitution reactions?
It’s often better to introduce halogens after establishing other substituents. This stepwise approach enhances regioselectivity.
Is it possible to get a pure meta or ortho/para product?
No, there will always be some mixture. Even a meta director like CF3 can produce up to 10% ortho/para products.




Leave a Comment