Reaction of Hydrogen Peroxide with Fire
Hydrogen peroxide reacts with fire by decomposing rapidly, releasing oxygen gas that intensifies combustion. This release of oxygen supports and accelerates burning, making hydrogen peroxide a strong oxidizer in the presence of flame or heat.
How Hydrogen Peroxide Decomposes Near Fire
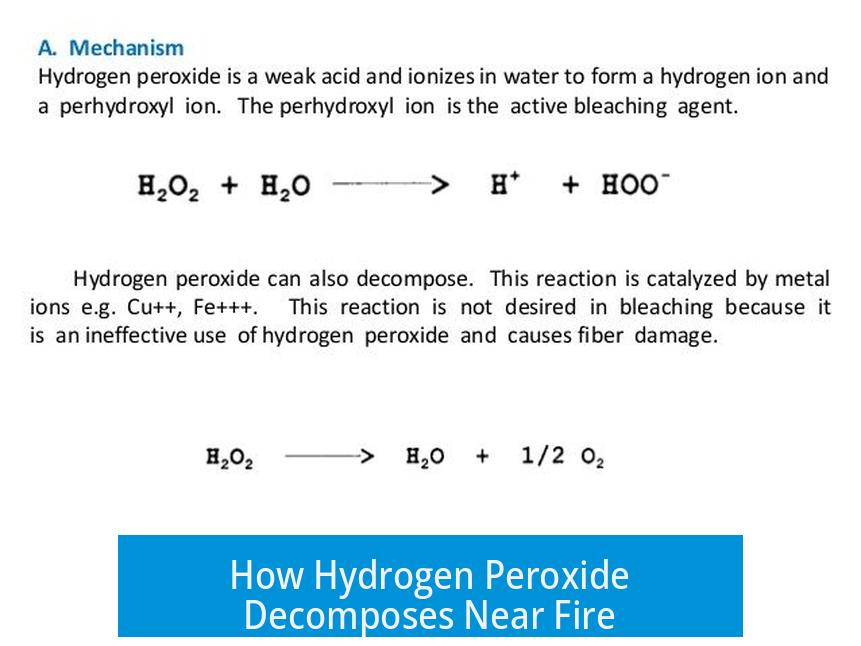
Hydrogen peroxide (H2O2) is unstable, especially when exposed to heat or catalysts. Near a flame, it decomposes as follows:
2 H2O2 → 2 H2O + O2
This reaction releases water and oxygen gas. The sudden release of oxygen increases the concentration of this oxidizer around the flame.

Role of Released Oxygen in Fire Behavior
- Oxygen supports combustion by reacting with fuel molecules.
- Higher oxygen levels lead to faster flame propagation.
- Intense oxygen release can cause flames to grow rapidly or flare up.
The increase in oxygen concentration from hydrogen peroxide decomposition makes fires more vigorous, even explosive in confined spaces or with high concentrations.
Concentration and Stability Considerations
Hydrogen peroxide’s behavior near fire strongly depends on its concentration:

| Concentration | Effect Near Fire |
|---|---|
| Low (3-6%) | Slow decomposition; mild oxygen release, less fire intensification |
| High (>30%) | Rapid oxygen release; heightened risk of flame acceleration or explosion |
Higher concentrations are reactive and dangerous near ignition sources.
Practical Implications

- Hydrogen peroxide should never be stored near open flames or heat sources.
- Spills may cause unexpected flares due to oxygen bursts.
- Contact with organic materials or impurities may catalyze rapid oxidation.
Understanding this reaction is important for handling, storage, and safety protocols to prevent fire hazards.
Summary of Key Points
- Hydrogen peroxide decomposes near fire to form oxygen and water.
- The released oxygen intensifies and accelerates combustion.
- Higher concentrations produce stronger and faster reactions.
- Safe handling avoids exposure to flames or heat to prevent fire risks.
The Fiery Dance: What Happens When Hydrogen Peroxide Meets Fire?
So, what actually happens when hydrogen peroxide reacts with fire? The straightforward answer is that hydrogen peroxide can dramatically accelerate combustion because it decomposes to release oxygen gas, feeding the fire like gasoline fuels a flame. This isn’t just a subtle effect; it can be a spectacularly fast and intense reaction depending on concentration and conditions.
Let’s break this down carefully—no science magic tricks here, just some neat chemistry you can imagine like a wild dance between molecules and flames.
Hydrogen Peroxide: The Behind-the-Scenes Oxygen Hero
Hydrogen peroxide (H2O2) looks innocent as a pale blue liquid, often found in medicine cabinets acting as a disinfectant. But beneath that calm facade lies a very reactive beast. The key to its fiery interaction is its tendency to decompose spontaneously:
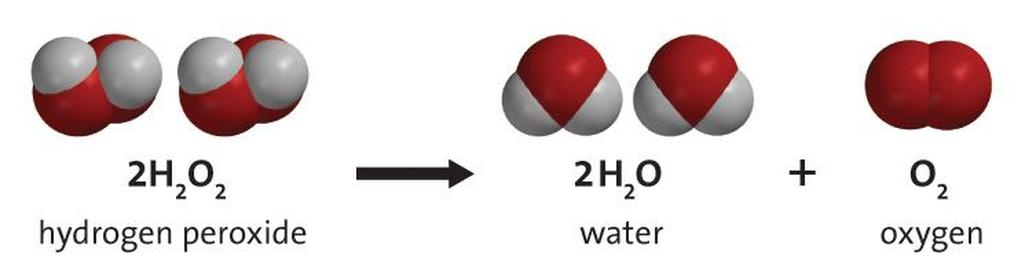
2 H2O2 → 2 H2O + O2
This means hydrogen peroxide breaks down into water and oxygen gas. That’s not just a boring after-school science reaction— it’s oxygen liberation on steroids. And oxygen is exactly what fire needs to thrive.
The amount of oxygen released and the speed of this decomposition depend largely on the concentration of the hydrogen peroxide. Higher concentration solutions (think 30% and above, sometimes used industrially) can release a ton of oxygen gas rapidly, fueling fires intensely.
The Fiery Reaction: How Oxygen Supercharges a Fire
Imagine you’re trying to light a campfire with damp wood. It smolders but struggles. Now, picture waving a bellows to blow oxygen into the coals—the fire suddenly catches and roars to life. The reaction of hydrogen peroxide with fire works like that bellows on a molecular scale.
When hydrogen peroxide encounters a flame or a hot surface, the heat triggers decomposition instantly. This abrupt burst of oxygen can cause flames to flare, intensify, or even spread unpredictably. The mixture of highly concentrated hydrogen peroxide and fire is an explosive combo that demands respect and caution.
In some demonstrations, applying concentrated hydrogen peroxide to combustible materials like wood or cloth can result in rapid flaming or even mini explosions. So yes, it “burps out a TON” of oxygen gas quickly. This oxygen jumpstarts combustion, turning small sparks into bright, roaring flames.
Why Don’t We See This Reaction More Often?
Good question! Most people encounter only very dilute hydrogen peroxide (around 3%), which decomposes much more slowly and does not release oxygen rapidly enough to intensify flames noticeably. It’s the concentrated stuff, often called “industrial strength,” that poses the fire hazard.
Another reason is that hydrogen peroxide is usually stored carefully in opaque bottles to prevent it decomposing prematurely under light or temperature changes. When it does start decomposing, you sometimes see bubbles of oxygen forming, but not flames—unless it meets a heat source.
Safety First: Handling Hydrogen Peroxide Near Fire
Here’s the practical takeaway: Mixing concentrated hydrogen peroxide and fire or heat sources isn’t a good idea unless you’re a trained chemist in a controlled environment. The rapid oxygen release can cause unexpected flare-ups and make fires grow suddenly out of control.
On the safety scale: small amounts of dilute hydrogen peroxide around a candle? Probably safe. A big splash of concentrated hydrogen peroxide on burning paper? Potentially catastrophic. Always read labels and use caution, or better yet, keep fire and reactive chemicals well apart.
Everyday Applications and Wild Reactions
Here’s an interesting twist—this oxygen-releasing property isn’t just a dangerous nuisance. It’s often harnessed constructively. For example, hydrogen peroxide is used in rocket propulsion systems and experimental toys called “chemical rockets.” The oxygen release propels them.
And in biology labs, hydrogen peroxide is used as an oxidizer and disinfectant, carefully controlled to prevent unwanted combustion. So, its reaction with fire is a double-edged sword—dangerous but also useful if handled smartly.
To Wrap Up: The Fiery Verdict
Hydrogen peroxide reacts with fire primarily by decomposing, producing oxygen gas that feeds flames, causing fire to intensify rapidly. The strength and speed of this reaction depend on the concentration of the hydrogen peroxide and the heat applied. Low concentrations fizz gently; high concentrations can fuel roaring fires or cause sudden flare-ups.
So next time you see hydrogen peroxide, remember: it’s not just a disinfectant hiding in your bathroom cabinet—it’s a potent oxidizer lurking ready to unleash oxygen at a moment’s heat. A fascinating chemical performer in the fiery dance of combustion!
Got curious how science turns simple chemicals into fiery spectacles? Feel like experimenting with chemistry safely at home or want to geek out over handling reactive substances? Drop your thoughts below or better yet, ask your local science expert! Because when hydrogen peroxide and fire meet, the chemistry gets HOT—and interesting.
Q1: What happens when hydrogen peroxide is exposed to fire?
Hydrogen peroxide can rapidly break down, releasing oxygen gas. This extra oxygen can make flames burn hotter and spread faster.
Q2: Does hydrogen peroxide itself catch fire?
Hydrogen peroxide is not flammable, but it supports combustion by releasing oxygen when heated or decomposed.
Q3: Why does oxygen release from hydrogen peroxide matter near fire?
Oxygen feeds fire. When hydrogen peroxide decomposes near flame, it supplies extra oxygen, increasing the fire’s intensity and size.
Q4: Can hydrogen peroxide cause explosions when heated or near fire?
High concentrations may release oxygen quickly and violently. This can cause pressure buildup or explosive reactions if confined.
Q5: How does hydrogen peroxide concentration affect its reaction with fire?
Stronger hydrogen peroxide solutions release more oxygen faster. This increases risk of rapid fire growth and hazards during heating or ignition.





Leave a Comment