Why Can a Simple Reaction Turn Tires into Liquid Fuel Despite Their Flammability?

Tires do not burn easily at everyday temperatures but require sustained heating to around 400°C for ignition. Despite this, a specialized chemical process called pyrolysis can break down tires into liquid fuel and other useful products. This process differs from simple burning because it involves controlled decomposition under heat and vacuum, enabling recovery of fuel-like liquids and valuable chemicals.
Understanding Tire Flammability and Ignition
Tires consist of complex rubber compounds, steel reinforcements, and various additives. They are combustible but have a high ignition threshold. To catch fire, a tire must be heated to approximately 400 degrees Celsius (750 degrees Fahrenheit) and maintained at that temperature for several minutes. This thermal resistance results from the vulcanized rubber and layered construction, which delays ignition despite combustible materials.
- Tires do not ignite spontaneously at ambient temperatures.
- Once ignited, they burn intensely.
- The ignition point is roughly 4 times the boiling point of water.
What is Tire Pyrolysis?
Tire pyrolysis is a thermochemical process. It involves heating shredded tire pieces in a vacuum or inert atmosphere without oxygen to prevent combustion. Instead of burning, the tire material decomposes into smaller molecules. This yields liquid fuel, gas, and solid residues.
| Step | Process Detail | Output |
|---|---|---|
| 1. Shredding | Separate steel wires, shred rubber into small pieces | Steel scrap for resale, fine rubber pieces |
| 2. Pyrolysis Heating | Heat rubber electrically under vacuum to ~400°C or higher | Breakdown of polymers into liquid tar and pyrolysis gases |
| 3. Product Recovery | Condensation and collection of pyrolysis tar and gas | Liquid fuel precursors, combustible gases |
| 4. Residue Collection | Solid residues (carbon black, soot) | Used as pigment/additive |
Chemical Complexity of Tires
Tires contain numerous components. Common passenger tires have up to 17 layers with various materials:
- Diverse rubber compounds (natural and synthetic)
- Fillers such as carbon black and silica
- Steel belts and polyester cords for strength
- Vulcanization agents like sulfur and curing chemicals
- Anti-oxidants and stabilizers to prevent degradation
This complexity makes de-vulcanization and clean breakdown difficult. Many chemicals resist decomposition or reform into unwanted compounds.
Challenges of Sulfur and Toxicity

Rubber vulcanization introduces significant sulfur content. This sulfur is difficult to remove during pyrolysis, leading to high sulfur levels in pyrolysis products. Sulfur-containing compounds can cause corrosive emissions and require additional cleaning or processing.
Toxic chemicals and incomplete combustion products pose environmental hazards. Burning tire-derived liquids directly releases pollutants harmful to air quality. Although pyrolysis converts tires into fuel-like materials, the resulting fuels are not suitable for vehicle engines without extensive refining.
Economic and Environmental Considerations
Pyrolysis requires substantial upfront investment (approximately $40 million for commercial plants) and high operational energy input. The process energy includes shredding, heating under vacuum, cracking products, and separation steps.
Fuel yield is modest. Around 70% by weight of a typical 12 kg car tire can theoretically become fuel. This translates to roughly 10 kg, equivalent to approximately 12 liters of gasoline by mass-to-volume conversion. Thus, recycling four tires yields about 48 liters, roughly one tank fill for a vehicle per 80,000 km. This low volume limits economic incentives.
Environmental costs include sulfur emissions, toxic by-products, and carbon dioxide release. Overall, burning pyrolysis liquids may produce net pollution without efficient emission controls.
Current Industrial Implementations
Despite challenges, several commercial pyrolysis plants operate globally, particularly in Europe, Asia, and Australia.
- Operators shred and pyrolyze tires at specialized facilities.
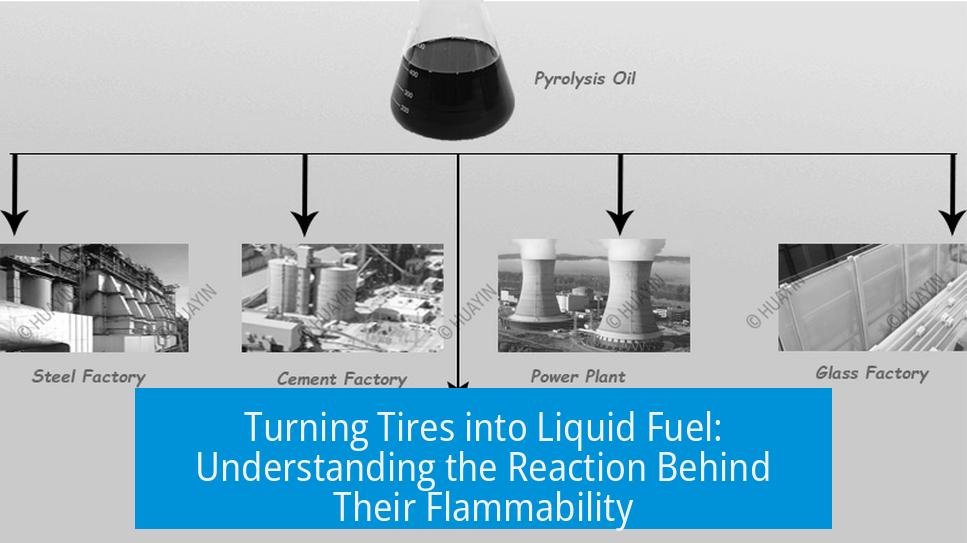
- Products include pyrolysis tar (chemical feedstock), pyrolysis gas (used internally for heating or electricity), and carbon black for reuse.
- Examples include a multi-module plant in New South Wales, Australia, with plans for expansion.
These plants demonstrate technological viability but face hurdles in scaling economically and minimizing pollution.
Alternative Tire Recycling Strategies
The majority of tire recycling does not involve fuel conversion. Instead, tires are:
- Processed into crumb rubber for playground surfaces or running tracks
- Used as fillers in asphalt or construction materials
- Repurposed for industrial products requiring rubber content
These methods consume less energy and reduce air pollution compared to pyrolysis-based fuel recovery. Governments and companies evaluate carbon taxes and incentives to improve waste tire management and encourage more sustainable approaches.
Summary of Key Points

- Tires ignite at about 400°C but require sustained heating for combustion.
- Pyrolysis thermally decomposes shredded tires under vacuum to produce liquid fuel precursors and other products.
- The multi-component nature of tires complicates breakdown and creates challenges with sulfur and pollutants.
- Burning pyrolysis liquids directly is environmentally harmful and fuels are not suitable for vehicles without further refining.
- Economic viability is limited by high energy input, modest fuel yield, and pollution control costs.
- Commercial pyrolysis plants exist but represent one option among less intensive recycling methods.
- Alternative uses such as crumb rubber recycling currently dominate tire waste management.
Tires Burn Easily, so Why Can a Simple Reaction Turn Them into Liquid to Be Used as Fuel?
At first glance, it seems straightforward: tires catch fire and burn easily, so why not just convert them into liquid fuel through a simple reaction? The honest answer is — it’s way more complicated than lighting a match. Despite tires being combustible, transforming them into a usable liquid fuel requires advanced technology, a hefty investment, and careful handling of hazardous by-products.
Let’s peel back the rubbery layers and explore the science, challenges, and real-world innovations surrounding the idea of turning tires into liquid fuel.
The Flammability Myth: Tires vs. Fire
Contrary to popular belief, tires are not exactly ready to burst into flames at a casual spark. They must be heated intensely to approximately 400°C (750°F) and kept at that temperature for minutes to ignite. It’s like persuading a stubborn pet to obey — tires resist fire at first.
This resistance comes from the complex makeup of tires. They are composed of multiple layers — some passenger tires have up to 17! These layers include various rubber types, steel wires, stabilizers, sicla, waxes, sulfur, and carbon black. More than a snack mix, this mix means they have poor thermal conductivity. When you put out a tire fire that looks doused, chances are, the interior is still smoldering and might reignite. This hidden burning complicates both safety and disposal.
Pyrolysis: The High-Tech Alchemy to Liquid Fuel
Here’s where the magic happens, but it’s not quite Hogwarts-level sorcery; it’s industrial engineering. Tire pyrolysis is the process of decomposing tires via heat in a vacuum, without burning them in oxygen. This high-tech method breaks down the tires into oil, gas, and solid residues.
- First, tires are shredded and steel wires removed for recycling.
- The rubber is further chopped into small pieces.
- Next, heat is applied electrically inside a special pyrolysis chamber under vacuum.
- The tire’s components break down, releasing pyrolysis tar and gas.
- These gases can fuel turbines that generate electricity to power the process.
- Remaining soot is collected as a high-grade industrial pigment, usually fed back into manufacturing new tires or plastics.
Commercial pyrolysis plants, especially in Europe, Asia, and now Australia, have proven this can work on a large scale — but it demands massive infrastructure costs, sometimes exceeding $40 million.
Why Not Just Burn Tires and Collect Fuel?

Sure, tires burn. They’re filled with hydrocarbons, so burning releases energy. But burning is messy and unhealthy. Tire fires emit toxic smoke laced with carbon monoxide, sulfur dioxide, cyanide, styrene, and other nasty chemicals. The process is incomplete and produces pollution detrimental to human health and the environment.
Burning tires outright dumps all trapped carbon back into the atmosphere with minimal energy efficiency. Also, the sulfur content in vulcanized tires is high, and removing sulfur compounds is a giant headache during pyrolysis and refining. This sulfur is linked to acid rain and further environmental harm.
So, burning tires for fuel isn’t a golden solution. It may generate heat but contributes heavily to pollution, making it a dirty and short-sighted choice.
Turning Tires Into Liquid Fuel: Reality Check on Yields
Let’s crunch some numbers for clarity:
| Metric | Value |
|---|---|
| Average passenger tire weight | ~11-12 kg |
| Fuel recoverable from tire | ~70% (about 8-10 kg) |
| Density of gasoline | 0.84 kg/L |
| Fuel volume per tire | ~12 liters of gasoline equivalent |
| Fuel from four tires (car’s set) | ~48 liters |
This fuel amount is roughly comparable to one tankful that can power a car for about 80,000 kilometers. Not bad, but not earth-shattering either when compared with the massive capital and operating expenditures. Scale matters. Plus, the complex composition and sulfur content make refining and usage challenging. Investors want quicker, bigger wins.
Environmental and Economic Challenges
Despite the technical feasibility, several hurdles curb enthusiasm from both environmental and economic perspectives.
Energy Cost: Turning tires into fuel isn’t a “free lunch.” Burning the tire directly releases pollution; pyrolysis requires significant electricity to heat the tires under vacuum, gas separation, cracking, and cleaning to meet fuel grade specs.
Pollution Concerns: Even after pyrolysis, the liquid fuel is not clean enough for vehicles directly due to leftover toxic compounds. Burning it for heat, like in industrial processes, remains a better case but still pollutes heavily.
Market Viability: The industry hasn’t widely embraced tire-to-fuel conversion, partly because recycling methods such as chopping tires for playground mulch or running tracks are simpler and cheaper.
There’s a need for government incentives or carbon taxes to tip scales toward tire pyrolysis plants. In places like Australia, companies like GDT are currently building multiple facilities to convert end-of-life tires into energy, showing progress but also highlighting the substantial effort required.
Alternative Solutions: More Than Burning
With all these problems, what else can be done with old tires besides shackling them to a giant bonfire?
- Recycling into Playground Mulch: Shredded tires provide cushioned surfaces, reusing waste safely.
- Running Tracks and Artificial Turf: Tire rubber granules serve as resilient base materials.
- Fuel for Cement Kilns: Some cement plants burn whole tires efficiently, capturing some of their energy in a controlled facility.
- Innovative Manufacturing Approaches: Some proposals involve using CO2 captured from the atmosphere to manufacture new tires, increasing sustainability instead of contributing more waste.
Compared to tire pyrolysis fuel production, these alternatives don’t require massive infrastructure and are less energy intensive.
The Takeaway: Tires Are Tough Nuts to Crack (and Burn)

Tires may seem like easy targets for burning, but their complex design and toxic ingredients pose tough challenges. While tire pyrolysis can turn rubber into liquid fuels, it’s no walk in the park. It demands high temperatures, specialized equipment, huge upfront investments, and careful environmental management.
Can they be a game-changer for sustainable fuel? Maybe, but currently, not without costs and risks. Perhaps the best path includes integrated approaches — smarter recycling, cleaner industrial uses, and investments in green chemistry converting CO2 into tire materials.
At the end of the road, tires tell us something important: nothing valuable comes easy, and quick fixes often come with a hefty price tag. So next time you see that rubber burning, consider the complex story behind it. It’s not just fire — it’s science, economics, and environmental responsibility all tied in a tough rubber bundle.
Have You Ever Witnessed a Tire Fire? What Do You Think About Turning Tires Into Fuel? Drop a comment below!
Why do tires require such high heat to ignite despite seeming flammable?
Tires need to reach about 400°C (750°F) and maintain that temperature for several minutes before they ignite. This is because their rubber resists heat and prevents quick combustion.
How can pyrolysis turn shredded tires into liquid fuel despite their complex composition?
Pyrolysis heats tire rubber in a vacuum, breaking it down into tar, gas, and soot. This controlled reaction transforms solid rubber into useful liquid fuels and other byproducts.
Why can’t we simply burn tire-based liquid fuel in vehicles?
Burning tire-derived liquid fuel produces toxic chemicals and poor combustion products. It harms air quality and is not suitable for vehicle engines.
What makes removing sulfur from pyrolyzed tire oil challenging?
High sulfur content comes from vulcanization additives in tires. Sulfur is difficult to extract, complicating refining and limiting fuel quality.
Is pyrolysis of tires economically viable on a large scale?
The process demands high energy input and costly infrastructure. Yield per tire is low, making it less profitable without subsidies or carbon credits.
What happens to the non-fuel parts of tires after pyrolysis?
Steel wires are recovered for resale, and leftover soot serves as pigment or additive in new tires, ensuring almost all material is reused.




Leave a Comment