Understanding the Periodic Table
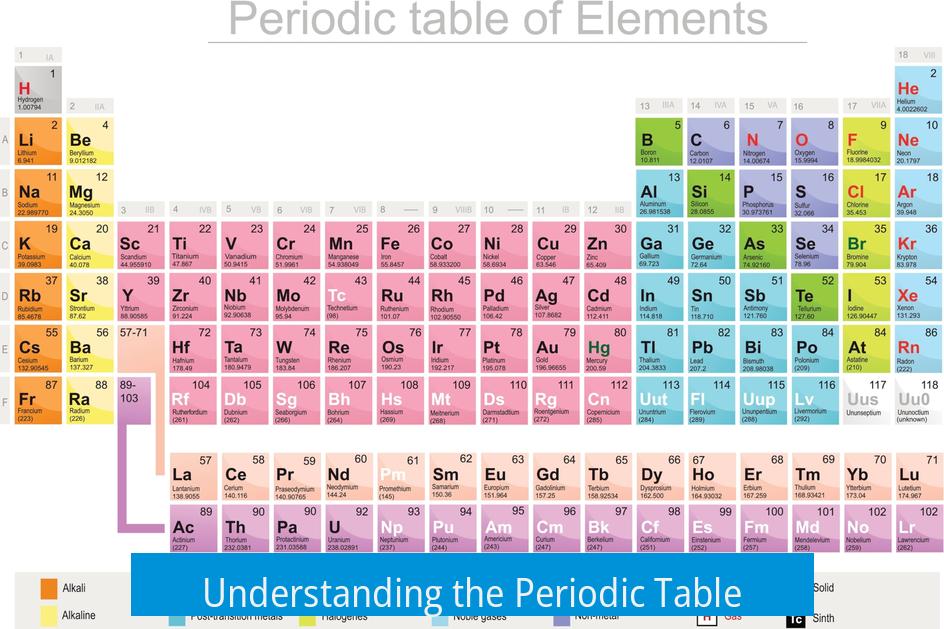 The periodic table organizes elements by their electronic structure, revealing patterns in their chemical properties and behaviors.
The periodic table organizes elements by their electronic structure, revealing patterns in their chemical properties and behaviors.
The table reflects how electrons fill atomic orbitals and shells. This arrangement governs element similarities and differences. Groups of elements share characteristics because they hold similar outer electron configurations.
Electronic Structure and Periodic Arrangement
Elements are arranged in periods and groups based on electron shells and subshells. Each period corresponds to filling a new electron shell, with periods featuring 2, 8, 18, or more elements depending on orbital capacity. This explains the periodicity of element properties.
- Groups (columns) contain elements with matching valence electron counts.
- Elements in the same group often show similar reactivity and characteristics.
- The shape of the periodic table stems from quantum mechanics principles governing electronic configurations.
Complexity Behind the Table
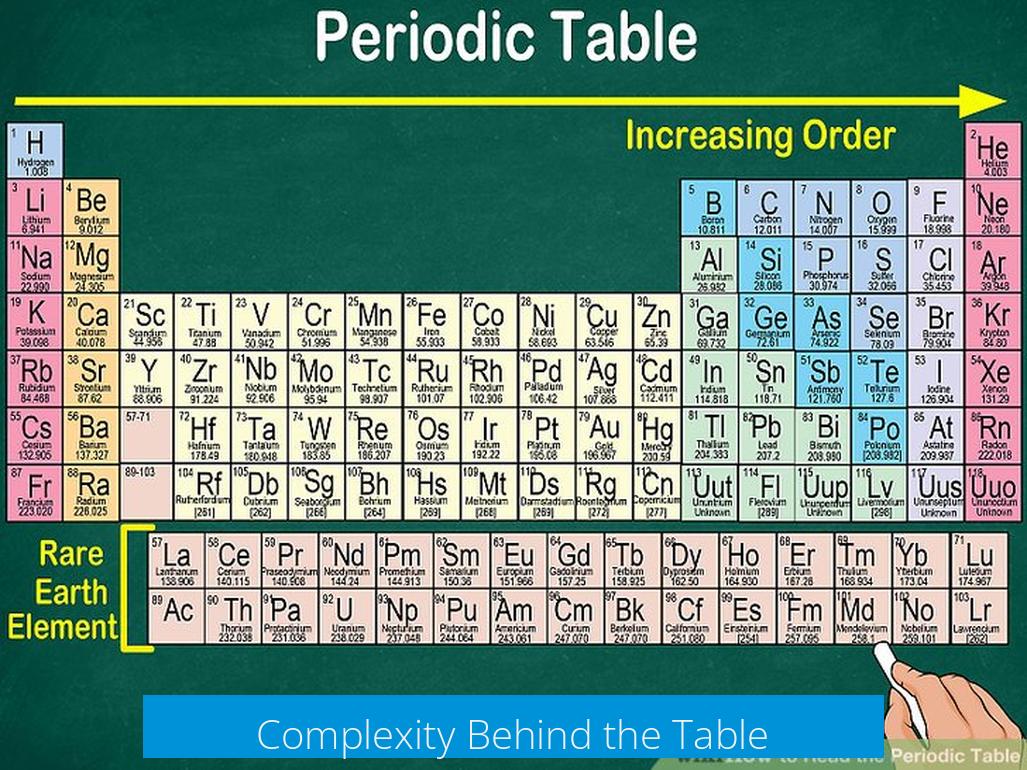
Even advanced chemistry students study the periodic table extensively. Full comprehension requires knowledge of quantum chemistry, a field often taught at the junior college level or higher. Many aspects of element behavior remain subjects of ongoing research.
Understanding the electronic structure grants insight into elemental properties without memorizing each element individually. Instead, learning foundational groups and key elements is more practical for beginners.
Effective Strategies to Start Learning
Starting with simpler elements provides a solid base:
- Focus first on elements hydrogen through fluorine, spanning nonmetals with varying properties.
- Learn alkali metals and alkaline earth metals together due to their shared characteristics.
- Transition metals like iron, copper, and zinc introduce more complex behaviors.
This approach allows gradual expansion to less common or more unusual elements, sparking deeper curiosity about the table.
Recommended Learning Resources
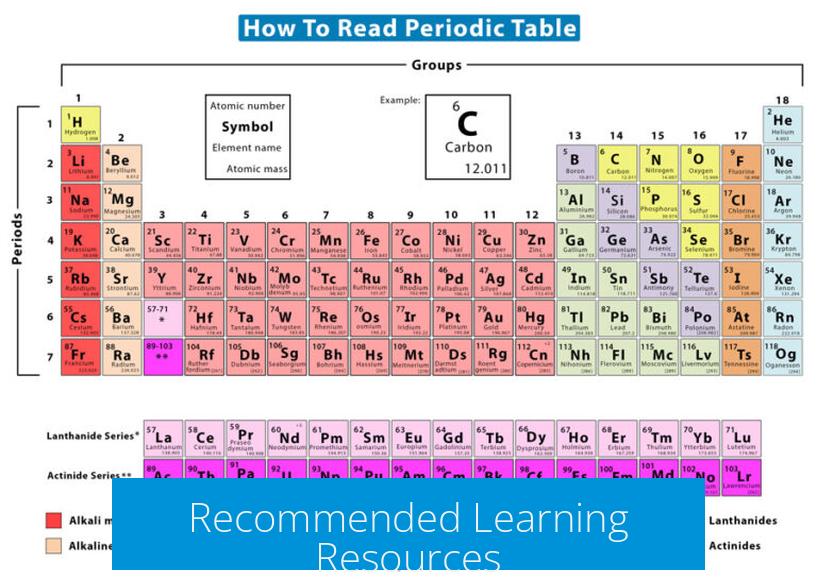
- Educational videos on the periodic table offer visual introductions.
- “The Elements” by Theodore Grey provides well-illustrated descriptions for general audiences.
- Wikipedia and other reputable scientific sources serve as comprehensive references.
Key Takeaways
- The periodic table’s layout mirrors elements’ electronic structures.
- Groups of elements exhibit similar chemical properties due to shared valence electrons.
- Learning in stages, starting with common elements, builds a strong foundation.
- Advanced understanding ties into quantum chemistry principles.
- Resources like illustrated books and videos enhance engagement.
Understanding the Periodic Table: Your Friendly Guide to Chemistry’s Ultimate Cheat Sheet
Understanding the periodic table starts with realizing it’s not just a chart — it’s a story about electrons, elements, and the universe’s quirky rules. At its core, the periodic table maps out elements based on their electronic structure, revealing why they behave the way they do. For anyone stepping into chemistry, embracing this view makes the periodic table much more than a list of symbols. It’s the secret language of matter.
If you’ve ever stared blankly at that grid of elements and wondered how to make sense of it without losing your mind, you’re in the right place. Let’s walk through the layers of complexity, starting points, and resources to turn that curiosity into clear understanding.
Why Is It So Complex? The Depth Beneath the Surface
The periodic table is a beast. Learning about its elements isn’t a weekend hobby—it’s a multi-year marathon for chemistry students. And even then, they barely scratch the surface!
Some elements are still shrouded in mystery, and understanding their full behavior often demands diving into the world of quantum chemistry, a branch usually tackled in junior college years or graduate studies. Yes, that means fancy math and physics concepts, like atomic orbitals and electron spin, start to play starring roles.
Want to truly get how elements behave? You might need to dust off physical chemistry or quantum mechanics textbooks, which explain electrons not just as particles but as waves, and how they organize themselves around nuclei. This electronic organization is the key to why the table looks the way it does.
Start Simple: Focus on the Essentials First
But don’t let that intimidate you. You don’t have to be a Ph.D. candidate to appreciate the periodic table’s beauty. Start small and smart.
Focus first on the first ten elements, say from Hydrogen to Fluorine. They each have distinct properties worth knowing. From there, explore groups like the alkali metals (think: lithium and sodium) and alkaline earth metals (like magnesium and calcium). They behave in ways that feel like chemistry’s version of family reunions—similar but with small, interesting differences.
Transition metals such as Iron (Fe), Copper (Cu), and Zinc (Zn) deserve a look, too. They often take center stage in real-world applications from construction to electronics. Suddenly, your casual curiosity might lead you to Xenon (Xe), a noble gas with surprising reactivity. It’s like chemistry surprises you when you least expect it.
Grab These Learning Tools: Your Periodic Table Sidekicks
Wondering where to begin? Here’s a starter pack for your journey:
- Check out this YouTube video for an entertaining introduction that makes elements approachable.
- Pick up The Elements by Theodore Gray. It’s beautifully illustrated and packed with fun facts, blending science with art. Don’t expect a textbook full of formulas; it’s more like a chemistry coffee table book that sparks curiosity.
- And of course, we can’t forget the grand encyclopedia of practically everything: Wikipedia. Dive into articles on elements and their properties whenever questions pop up (and they will!).
Unlocking the Periodic Table with Electronic Structure
Here’s the magic trick: The periodic table is basically a map reflecting electronic structure. When you learn how electrons nestle into shells and atomic orbitals around the nucleus, everything begins to click.
The shape of the periodic table is far from random. Those funny periods with 2, then 8, then 18 elements? They’re a direct consequence of how many electrons can fit in each shell.
Groups that run down the table share remarkably similar chemical properties because their outer electron arrangements are alike. This explains why elements in the same family, like the halogens or alkali metals, behave like a close-knit crew.
Stop trying to memorize element placement as if it’s a phonebook. Instead, understand why elements hang out where they do, and you’ll never confuse Chlorine (Cl) with Neon (Ne) again.
A Quick Recap and a Challenge
So, what have we unpacked? The periodic table is a layered puzzle:
- It’s a deep field that’s still being explored by scientists today.
- Start learning with common and simpler elements to build confidence.
- Use engaging resources to make the journey fun and less daunting.
- Understand electron arrangements to truly grasp the table’s order.
Here’s a question for you: If elements are defined mostly by their electrons, what happens when we alter these electrons through reactions or in excited states? How does that shape the world around us? Exploring this can lead from the periodic table all the way to new technologies and materials.
Understanding the periodic table isn’t just an academic chore. It’s the key to decoding the material world, a way to anticipate how substances behave, and yes, a way to impress your friends at parties when you casually drop, “Did you know Xenon can be reactive under the right conditions?”
So, why not start exploring now? The periodic table is more than just columns and rows. It’s a gateway to the elements of life, technology, and the cosmos code.




Leave a Comment