What Causes an Atom to Lose or Gain an Electron and Become an Ion?
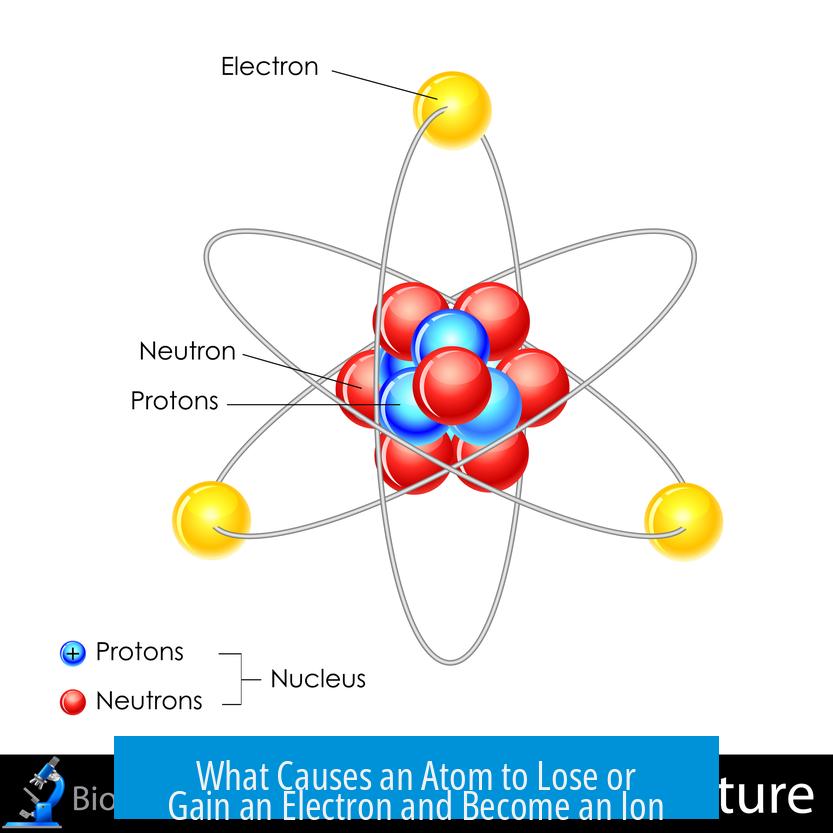
Atoms lose or gain electrons to achieve greater stability by completing their outer electron shells, often during chemical interactions with other atoms. This electron transfer results in charged particles called ions. Metals, for instance, tend to lose electrons and form positive ions, while nonmetals often gain electrons to form negative ions.
Why Do Atoms Transfer Electrons?
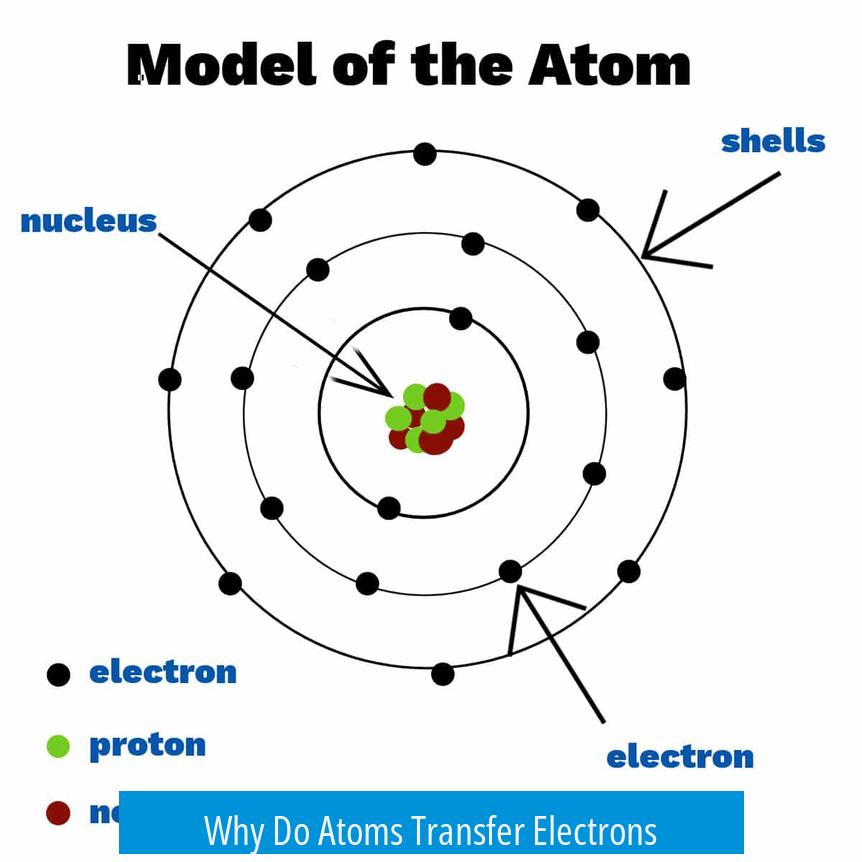
Atoms seek a stable electron arrangement, usually a filled outer shell like that of noble gases. Losing or gaining electrons helps them reach this goal. They do not randomly lose or gain electrons; rather, electron transfer occurs during bonding to minimize energy.
- Metal atoms lose electrons to form positive ions in metallic bonding.
- Covalent molecules share electrons instead of transfer.
- Salts form by the transfer of electrons between metals and nonmetals, creating ions.
Electron exchange only happens when an atom can interact with another atom capable of accepting or donating electrons. This interaction resembles a mutual agreement between the atoms to exchange electrons to achieve stability.
Where Do Gained Electrons Come From?
When an atom gains an electron, the electron comes directly from another atom involved in the reaction. For example, in the reaction between lithium and chlorine:
2 Li + Cl2 → 2 LiCl
Lithium atoms lose electrons to chlorine atoms during the reaction. The lithium atoms become positive ions by donating electrons. Chlorine atoms gain these electrons, becoming negative ions. Thus, electrons are transferred directly from one atom to another during chemical reactions. No isolated free electrons exist; electrons always move from one atom’s outer shell to another’s.
Summary of Key Points
- Atoms become ions by losing or gaining electrons to reach stable electron configurations.
- Electron transfer occurs through chemical bonding, influenced by atom types and their interactions.
- Gained electrons come from other atoms during reactions, never existing freely.
- This electron exchange drives the formation of ionic compounds like salts.
Why do atoms lose or gain electrons to form ions?
Atoms lose or gain electrons to reach a stable state. This stability often means having a full outer shell. It helps them form bonds and lowers their overall energy.
What drives one atom to give up electrons while another accepts them?
Atoms do not lose or gain electrons alone. They interact with other atoms. The donating atom “agrees” to give up an electron if it improves stability for both.
When an atom gains an electron, where does that electron originate?
The electron comes from another atom involved in the reaction. For example, in lithium chloride formation, lithium loses electrons, which chlorine gains.
Do all atoms easily lose or gain electrons?
No, not all atoms do this easily. The ease depends on the atom’s properties and the surrounding atoms it interacts with.
How do different types of bonding relate to electron transfer?
Metals use metallic bonding, where electrons move freely. Covalent bonds share electrons. Ionic bonds happen when atoms transfer electrons entirely.



Leave a Comment