What Causes Chemical Bonds?
Chemical bonds form due to the lowering of energy when atoms share or exchange electrons, leading to more stable arrangements. Atoms achieve stability by creating bonding orbitals that hold electrons at lower energy levels compared to when they are isolated. This energy change drives bond formation.
Energy Lowering and Stability
Atoms bond to attain a state with lower energy. For example, two hydrogen atoms, each with one electron, combine their electrons into a bonding molecular orbital. This orbital is less energetic than separate atomic orbitals, increasing the system’s stability. The process releases energy, which means the bond must be broken by re-adding that energy.
Molecular Orbital Theory
When atoms approach, their atomic orbitals overlap and form molecular orbitals. These new orbitals can hold electrons that are spread over the whole molecule rather than localized on one atom. Electrons fill orbitals according to rules—only two electrons per orbital with opposite spins.
| Orbital Type | Electrons Allowed | Description |
|---|---|---|
| s | 2 | Spherical orbitals close to the nucleus |
| p | 6 | Three dumbbell-shaped orbitals per shell |
| d | 10 | Five complex-shaped orbitals |
| f | 14 | Seven even more complex orbitals |
A filled shell comprises a fully occupied set of these orbitals, granting extra stability. Molecular orbital theory explains chemical bonds by detailing how electrons occupy these orbitals in molecules. Learn more.
Electron Arrangement and Shell Completion
Atoms become stable by filling their outer electron shells. Electrons are drawn to the nucleus by its positive charge but repel each other. For example, fluorine needs one electron to complete its outer shell. This addition is highly favored because the tightly charged nucleus attracts that electron strongly.
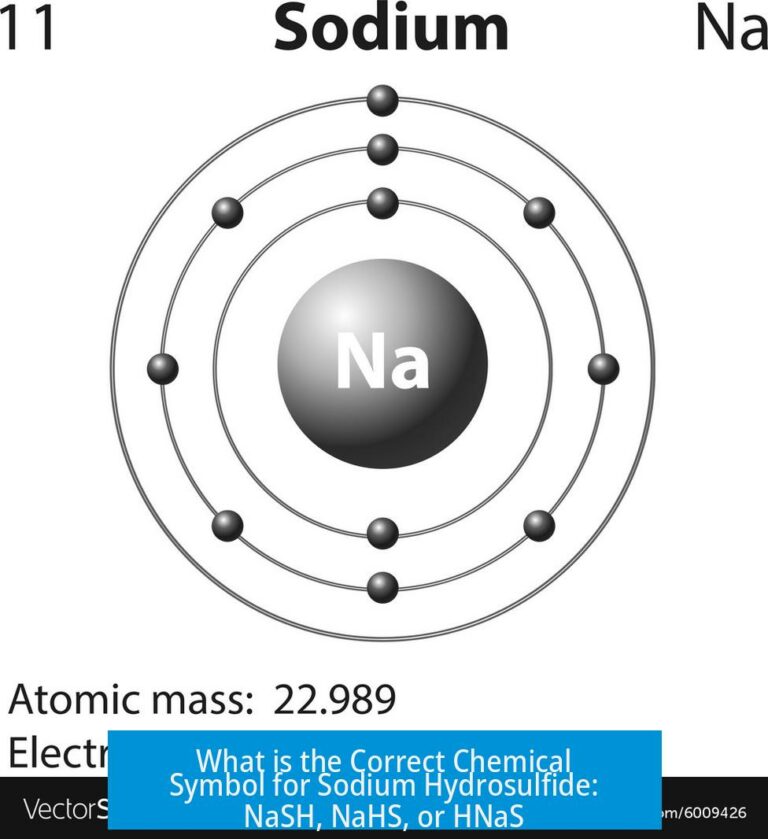
In contrast, sodium’s outer electron is shielded by inner shells and is further from the nucleus. This electron does not bind tightly and can be easily lost, indicating why sodium tends to form positive ions.
These examples show how electron positions and shell completion influence bonding tendencies. More details are available here.
Magnetic Attractions and Repulsions
At the fundamental level, chemical bonds result from magnetic-like attractions and repulsions between electrons and nuclei. The balance of these forces determines how electrons arrange themselves and how atoms bond.
Key Takeaways
- Chemical bonds form to lower the energy and stabilize atoms by sharing or transferring electrons.
- Molecular orbitals result from atomic orbital overlap and dictate electron arrangement in bonds.
- Full electron shells offer enhanced stability, driving atoms to gain, lose, or share electrons.
- Electron behavior is influenced by nuclear charge, distance, and electron repulsion forces.
- Magnetic attraction and repulsion underlie the forces governing bond formation.
What causes atoms to form chemical bonds?
Atoms form chemical bonds to lower their energy and increase stability. When atoms come together, they release energy and create a bonding orbital with less energy than separate atoms.
How does electron arrangement affect bond formation?
Electrons fill specific orbitals and shells. Atoms tend to bond to fill or empty their outer shells, achieving a more stable electron arrangement.
Why do atoms release energy when bonding?
Energy is released because bonded atoms reach a lower energy state. Breaking the bond requires adding energy back to separate them.
What role does the nucleus’s charge play in bonding?
The attraction between electrons and the positive nucleus influences bonding. Electrons are drawn closer to the nucleus, especially when a shell is nearly full.
How does molecular orbital theory explain bonding?
Molecular orbital theory shows that atoms create shared orbitals combining electrons. These bonding orbitals have lower energy, which stabilizes the molecule.



Leave a Comment