Understanding the Difference Between Specific Latent Heat of Fusion and Enthalpy of Fusion
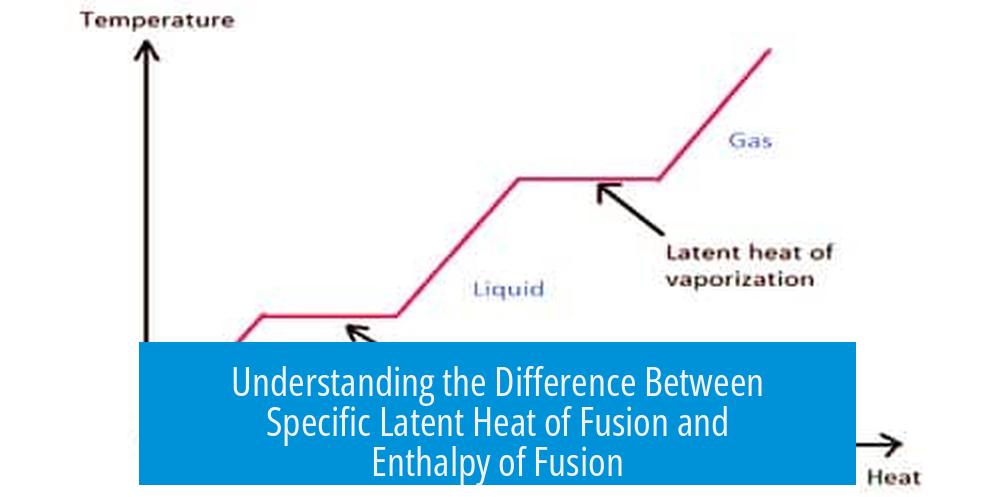
The specific latent heat of fusion and enthalpy of fusion refer to related thermodynamic quantities that describe the energy involved in a phase change from solid to liquid, but they differ primarily in their reference basis and measurement context. Specific latent heat is an intensive property defined per unit mass (or mole), while enthalpy of fusion typically denotes an extensive property linked to total heat absorbed or released under constant pressure. Both represent energy changes during melting, but choosing between terms depends on the context and measurement conditions.
Basic Definitions and Units

- Specific latent heat of fusion refers to the energy required to change a unit mass of a substance from solid to liquid at constant temperature and pressure without changing its temperature. It is expressed in units like joules per gram (J/g) or joules per mole (J/mol).
- Enthalpy of fusion
In thermodynamics, the qualifier “specific” means per unit mass or mole, making it an intensive property, which does not depend on the amount of substance. Enthalpy itself is an extensive property that scales with substance amount.
Thermodynamic Context and Measurement Conditions

The thermodynamic definition of enthalpy (H) is crucial for understanding enthalpy of fusion:
H = U + PV
where U is internal energy, P is pressure, and V is volume.
For phase transitions such as fusion, measurements usually occur at constant pressure (isobaric conditions). Under such circumstances, the heat exchanged (q) with the surroundings equals the change in enthalpy (ΔH) because no non-pressure work occurs beyond expansion work.
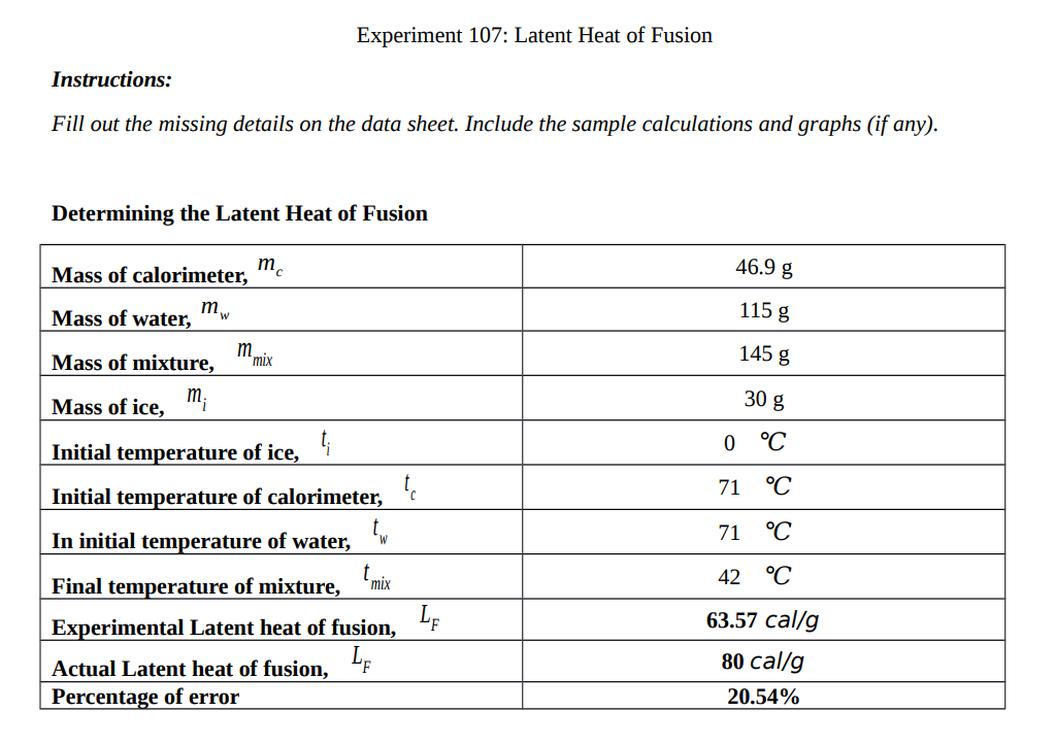
- At constant pressure, q = ΔH.
- When conditions deviate from constant pressure, heat and enthalpy are no longer directly equal. Calculations separate heat flow and work effects.
Most experimental determinations of latent heat involve calorimetry at atmospheric pressure, implying enthalpy of fusion and latent heat of fusion refer to the same measured energy change. This practical approach explains why these terms are often used interchangeably in literature and instruction.
Equivalence and Interchangeability of Terms

In many educational and practical contexts, the specific latent heat of fusion and specific enthalpy of fusion are treated as synonyms:
- Both describe the energy needed to melt one unit of mass without temperature change.
- Both assume constant pressure during the measurement.
This interchangeability holds true as long as the experimental or theoretical assumptions include constant pressure and no other work terms complicate the energy balance.
When Is One Term Preferred Over the Other?
The choice between “specific latent heat of fusion” and “enthalpy of fusion” depends on:
- Measurement Method: If the process is monitored using calorimetry in an open system at constant atmospheric pressure, enthalpy of fusion is directly measured and thus preferred in thermodynamic analyses.
- Amount Considered: For bulk or sample-wide energy changes, use “enthalpy of fusion,” which is an extensive quantity measured in joules or kilojoules. For per-unit mass or mole descriptions, use “specific latent heat of fusion.”
- System Constraints: Under non-isobaric or closed conditions (e.g., isochoric), heat may differ from enthalpy change. Then, enthalpy of fusion must be carefully computed using thermodynamic relations instead of simple heat measurements, making the term “enthalpy” more accurate.
- Academic vs. Application Settings: Educational contexts often treat the terms synonymously, while research requiring precision distinguishes them based on conditions and quantity definitions.
Summary Table of Differences
| Aspect | Specific Latent Heat of Fusion | Enthalpy of Fusion |
|---|---|---|
| Definition | Energy per unit mass/mole needed to melt solid without temperature change | Total enthalpy change during melting at constant pressure |
| Units | J/g, J/mol (intensive) | J, kJ (extensive) |
| Property Type | Intensive (normalized) | Extensive (total energy) |
| Measurement Condition | Often idealized, per unit mass basis | Measured via calorimetry at constant pressure |
| Thermodynamics | Represents latent heat per unit | Represents enthalpy change, equals heat only if isobaric |
| Common Usage | Material property data tables, comparisons | Thermodynamic and calorimetric data reporting |
Practical Recommendations for Usage
- In material science, specifying the “specific latent heat of fusion” clarifies energy per unit mass needed for melting, useful for engineering calculations.
- In thermodynamic discussions about energy changes under constant pressure, “enthalpy of fusion” provides a rigorous and directly measurable quantity.
- For students beginning thermodynamics, treating the two terms as equivalent is acceptable when constant pressure and simple phase changes apply.
- In complex or non-isobaric experimental setups, explicitly distinguishing enthalpy from heat flow or latent heat avoids confusion and ensures accurate energy accounting.
Key Takeaways
- Specific latent heat of fusion is the energy per mass or mole required for melting; enthalpy of fusion is the total enthalpy change for the process at constant pressure.
- They describe the same physical transition but differ in scale (intensive vs. extensive) and thermodynamic definitions.
- Enthalpy of fusion equals heat absorbed only under isobaric conditions; otherwise, corrections are needed.
- Use “specific latent heat” when focusing on material properties; prefer “enthalpy of fusion” in calorimetric or thermodynamic analyses.
- In most practical and educational cases, terms can be considered interchangeable if contextual assumptions hold.
What’s the Difference Between Specific Latent Heat of Fusion and Enthalpy of Fusion? And When Should You Pick One Over the Other?
Let’s answer this straight away: Specific latent heat of fusion and enthalpy of fusion essentially describe the same physical process—the energy needed to turn a solid into a liquid at its melting point—but with a subtle difference in units and usage context. They often overlap and can be interchangeable, but understanding when to use which term can save your sanity in chemistry or physics class, and in real lab work.
So, what causes the mildly confusing fuzz around these terms? And when does it matter? Keep reading, and you’ll see it’s less about “which is right” and more about “what fits best.”
Decoding the ‘Specific’ in Specific Latent Heat of Fusion
The word “specific” often sneaks into thermodynamics to mean “per unit something” — usually per unit mass, or sometimes per mole. When you hear specific latent heat of fusion, think: “energy needed to melt one gram (or one mole) of the substance.” Your units look like joules per gram (J/g) or joules per mole (J/mol), not the big total of joules.
In contrast, latent heat of fusion or just enthalpy of fusion can be about the entire system or sample, measured in joules (J). It’s an extensive property, meaning it depends on how much you have.
Why does this matter? Because being “specific” makes data more flexible. You can scale up or down without recalculating from scratch.
Unpacking Enthalpy of Fusion: What’s the Magic Thermodynamics Behind the Term?
Enthalpy, a fancy word often shortened as “H,” is a state function in thermodynamics. It’s defined as:
H = U + PV
Where U is internal energy, P pressure, and V volume. The big takeaway: enthalpy combines the system’s energy and the work it might do to push against surrounding pressure.
For fusion happening at constant pressure (which is typical—imagine melting ice in open air), the change in enthalpy equals the heat absorbed by the system. So, the enthalpy of fusion is the heat needed to convert a solid to liquid at constant pressure.
In controlled lab experiments using calorimetry, enthalpy can be measured directly because pressure doesn’t change much during melting. But in systems where pressure varies, the picture gets messier—enthalpy change no longer equals heat transfer directly.
When Are These Terms Interchangeable? Spoiler: Most of the Time!
Many textbooks and scientific discussions treat specific latent heat of fusion and enthalpy of fusion as synonyms. If a problem doesn’t state otherwise, you can assume they mean the same thing—especially if the pressure is constant and the process is adiabatic (no heat exchange with the environment besides the melting process itself).
For example, the energy it takes to melt 1 gram of ice can be called its specific latent heat of fusion or its enthalpy of fusion per gram. Both describe the 334 J/g needed to break hydrogen bonds and shift ice into water.
But There Are Exceptions—Enter the Lab Geek Mode
When does it get hairy? When the process isn’t straightforward, like:
- Isochoric conditions: Constant volume experiments where pressure may vary. Here, enthalpy and heat exchanged don’t line up perfectly because work done by the system changes.
- Non-constant pressure processes: If pressure changes during melting, you can’t directly equate heat measured to enthalpy changes without extra calculations.
- Complex mixtures or phase transitions under pressure: Enthalpy defines the thermodynamic quantity, but latent heat might be an experimental value approximated or corrected.
In these cases, understanding which term to use avoids confusion. Enthalpy is more fundamental thermodynamically, while latent heat often describes practical heat measured or exchanged.
So, When Should You Use Specific Latent Heat of Fusion or Enthalpy of Fusion?
If you want a simple, mass-normalized number to compare substances or scale heating/cooling calculations, use specific latent heat of fusion. It gets you per gram or per mole energy needs without fuss.
If you deal with actual thermodynamic properties under precise conditions, especially involving calculations with pressure and volume, turn to enthalpy of fusion. It’s more rigorous and fits well with state functions and thermodynamic cycles.
Hemoglobin melting? Use enthalpy. Melting an ice cube in a pot? Specific latent heat will likely do the trick.
Don’t get boxed in. Interpretation depends on context. The difference is more about the perspective—extensive versus intensive property—rather than conflicting definitions.
Practical Example That Shows the Difference
Suppose you’re a student and see this question:
“Calculate the energy required to melt 500 g of ice at 0°C.”
If you use specific latent heat of fusion, you multiply 334 J/g by 500 g and get 167,000 J. Simple and to the point.
If the question says “enthalpy of fusion,” you must clarify if this means per mole or total system enthalpy change. If it’s per mole, convert grams of ice to moles first, then multiply by molar enthalpy of fusion (about 6.01 kJ/mol). The answer ends the same but relays thermodynamic rigor.
Summary: Making Sense of These Thermodynamic Twins
- Specific latent heat of fusion refers to the heat required per unit mass (or mole) to melt a substance.
- Enthalpy of fusion is a thermodynamic state function describing heat absorbed under constant pressure conditions during melting.
- They are mostly interchangeable in everyday use, particularly under typical lab or classroom isobaric environments.
- Choose specific latent heat when dealing with practical, mass-normalized heat energy calculations.
- Choose enthalpy of fusion when thermal system rigor and pressure-volume work considerations come into play.
Choosing between them isn’t about right or wrong but about matching the term to your context. Think of it like picking the right screwdriver: Phillips or flathead? Both loosen screws, but one fits better depending on the screw.
In conclusion, the next time someone throws these terms at you, smile knowingly and say, “It depends—but I’m ready for both!”
What does the term “specific” mean in specific latent heat of fusion?
“Specific” means per unit mass or per mole. Specific latent heat of fusion is the heat required to melt one gram or one mole of a substance. It is an intensive property, unlike total latent heat, which depends on the amount.
Are specific latent heat of fusion and enthalpy of fusion the same?
They are often used interchangeably if the units and context are clear. Both describe the heat absorbed during melting at constant pressure, so in many cases, they represent the same physical quantity.
When is enthalpy of fusion equal to the heat measured during melting?
Enthalpy of fusion equals the heat absorbed only under constant pressure (isobaric) conditions. If pressure changes, heat and enthalpy may not be equal, and corrections are necessary.
When should one prefer using enthalpy of fusion instead of specific latent heat?
Use enthalpy of fusion in thermodynamic calculations involving pressure, volume, and energy changes. Specific latent heat is better for practical measurements on a mass basis, such as in lab experiments.
How do experimental conditions affect the choice between specific latent heat and enthalpy of fusion?
In ideal or constant-pressure setups, both terms are equivalent. In non-isobaric or varying-volume processes, enthalpy changes differ from direct heat measurements, so enthalpy is more accurate to use there.


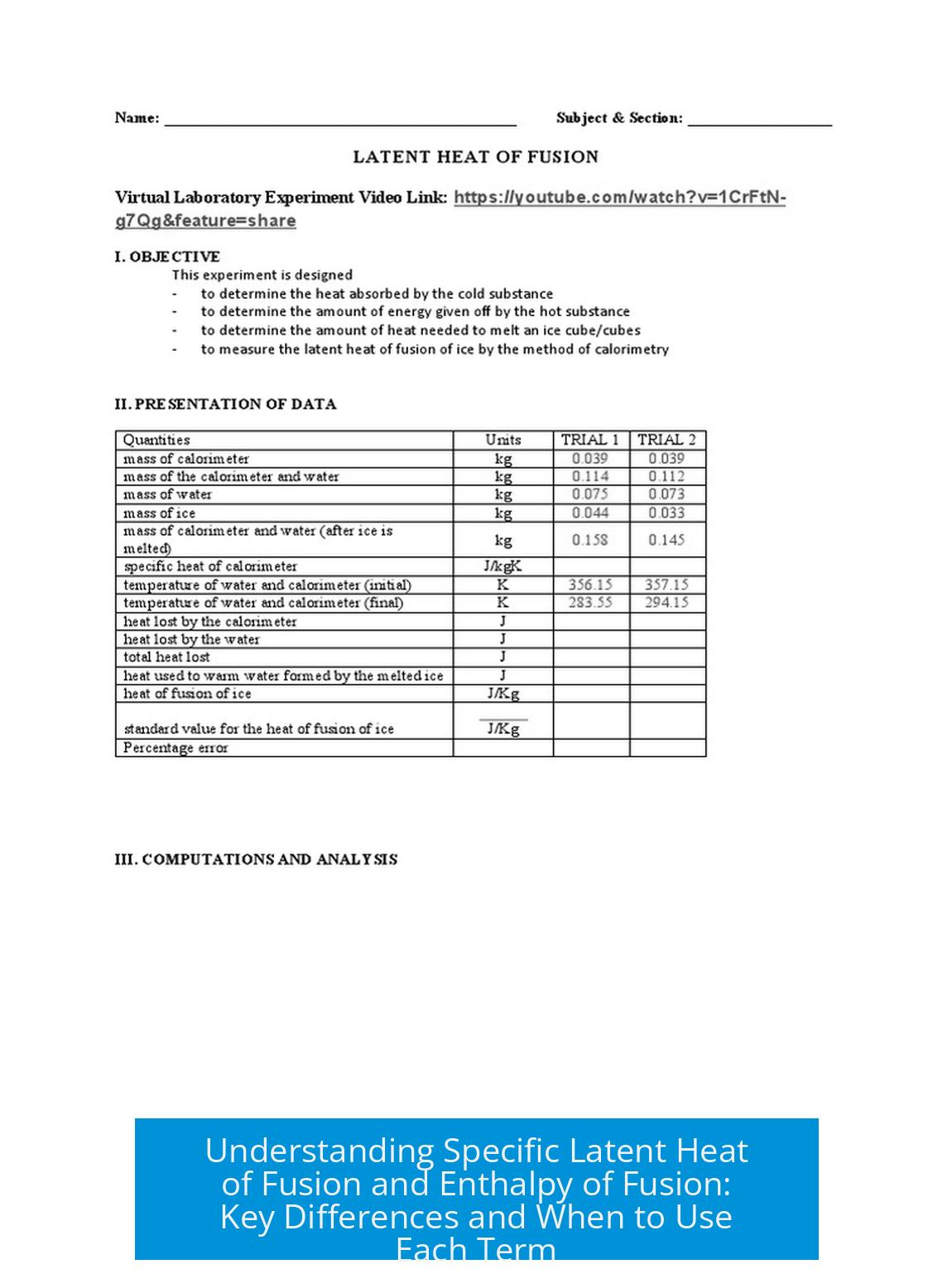


Leave a Comment