Atomic mass is an important concept in physics and chemistry and can be used to calculate the mass of a single atom or group of atoms. But what is atomic mass and how do you calculate it? Understanding atomic mass requires an understanding of the components of an atom, such as the protons, neutrons, and electrons that make up its nucleus. To calculate the mass of a single atom of an element, you must take into account the number of protons and neutrons present in its nucleus.
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. This mass is typically very close to the mass number, which is the total number of protons and neutrons in an atom, although there can be some variation. Knowing the atomic mass of an atom or group of atoms can be used to determine their chemical and physical properties, such as their boiling point or reactivity.
There are several methods to calculate the atomic mass of atoms or groups of atoms. One such method is to reference the periodic table, where an atomic number is indicated under the representation of an element. This number is usually close to the mass number, and by adding the masses of each proton and neutron present in the nucleus of a given atom, you can calculate the atomic mass. Another method of calculating the atomic mass is to use isotope data, which involves measuring the mass of naturally occurring isotopes of the same element.
Knowing how to calculate atomic mass is essential for understanding the physical and chemical properties of atoms and groups of atoms. By understanding the components of an atom and the methods used to calculate its mass, you can gain a better understanding of the fundamental building blocks of the universe.
How to calculate atomic mass?
Atomic mass is an important concept in chemistry and physics, and you may be asked to calculate it at some point. The method you use to find the atomic mass of an element will depend on the information you have. Before you start, it’s important to understand what atomic mass is and how it is calculated.
What Is Atomic Mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass in a group of atoms. It is also sometimes referred to as the atomic weight. Atomic mass is an important concept in chemistry and physics, and it can be used to calculate the relative mass of molecules, the amount of energy released in a chemical reaction, and more.
How to Calculate Atomic Mass
There are three different methods to calculate atomic mass: by referring to the periodic table, by adding the masses of the protons and neutrons, or by using the mass spectrometer.
By Referring to the Periodic Table
The periodic table is a chart of elements arranged by atomic number, which is the number of protons in the nucleus of an atom. It also includes the atomic mass for each element, which is usually very close to the mass number (the total number of protons and neutrons in the nucleus).
By Adding the Masses of the Protons and Neutrons
In order to calculate the mass of a single atom of an element, you can consider the number of protons and neutrons present in its nucleus. By adding the mass of each proton and neutron, you can get the atomic mass of that atom. This can be a time-consuming process, so it’s usually only used for small numbers of atoms.
By Using the Mass Spectrometer
The mass spectrometer is a device used to measure the mass of atoms. It works by ionizing atoms (removing electrons) and then measuring the mass of the resulting ions. The mass spectrometer is the most accurate way to measure atomic mass, but it is also the most expensive and complex method.
Once you understand how to calculate atomic mass, you can use it to answer questions about chemistry and physics. It’s an important concept to understand and can be used to calculate the relative masses of molecules, the amount of energy released in a chemical reaction, and more.
Whether you’re studying chemistry or physics, understanding how to calculate atomic mass is a valuable skill. With the right information and methods, you can determine the atomic mass of any element.
How do you find the mass number?
The mass number of an atom or isotope is the sum of the number of protons and the number of neutrons in its nucleus. It is an important value that helps us to identify different types of atoms and isotopes.
The best place to look for an element’s mass number is in the periodic table. It is displayed under the symbol for the element. By knowing the mass number of an atom, we can determine the number of protons and neutrons in the nucleus.
What is the mass number?
The mass number of an atom is the sum of the number of protons and the number of neutrons in the nucleus. It is also known as the nucleon number, since it is the total number of nucleons (protons and neutrons) in the nucleus.
The mass number of an atom is usually written as a superscript to the left of the element’s symbol. For example, the mass number of carbon-12 is written as 12C.
How to calculate the mass number?
The mass number of an atom can be calculated by adding the number of protons (atomic number) and the number of neutrons. For example, the mass number of carbon-12 is calculated as follows:
Mass number = Atomic number + Number of neutrons
Mass number of carbon-12 = 6 + 6 = 12
The atomic number of an element is the number of protons in its nucleus, and the number of neutrons can be calculated by subtracting the atomic number from the mass number. For example, the number of neutrons in carbon-12 is calculated as follows:
Number of neutrons = Mass number – Atomic number
Number of neutrons in carbon-12 = 12 – 6 = 6
What is the difference between mass number and atomic mass?
The mass number is the sum of the number of protons and the number of neutrons in an atom. The atomic mass is the average mass of an atom of an element. It is the sum of the masses of the protons and neutrons, weighted by the abundance of each isotope.
The atomic mass is usually written as a subscript to the right of the element’s symbol. For example, the atomic mass of carbon-12 is written as 12C.
The mass number of an atom or isotope is the sum of the number of protons and the number of neutrons in its nucleus. It is an important value that helps us to identify different types of atoms and isotopes. The mass number of an atom can be calculated by adding the number of protons (atomic number) and the number of neutrons. The atomic mass is the average mass of an atom of an element, and is usually written as a subscript to the right of the element’s symbol. Knowing the mass number and the atomic mass of an atom can help us to determine the number of protons and neutrons in its nucleus.
What is atomic mass and how is it calculated?
Atomic mass is a fundamental property of all atoms. It is the sum of the masses of its protons, neutrons, and electrons, and it determines the element’s atomic weight. Atomic mass is usually measured in atomic mass units (amu). Knowing the atomic mass of an element is essential to understanding its physical and chemical properties.
What Is Atomic Mass?
Atomic mass is the total mass of the protons, neutrons, and electrons in an atom or group of atoms. It is usually expressed in atomic mass units (amu). The atomic number (Z) is the number of protons in the nucleus of an atom and the mass number (A) is the total number of protons and neutrons in the nucleus. The sum of both the atomic number and the mass number for an atom (A-Z) corresponds to the total number of subatomic particles present in the atom.
How to Calculate Atomic Mass
There are three different methods to calculate the atomic mass of an element. The first is to use the periodic table. Most elements have their atomic mass listed in the periodic table. For example, the atomic mass of oxygen is 16.00 amu.
The second method is to use the mass number and the atomic number. The mass number is the sum of the number of protons and neutrons in the nucleus, while the atomic number is the number of protons in the nucleus. The atomic mass is calculated by subtracting the atomic number from the mass number. For example, the atomic mass of carbon-12 is 12.00 amu.
The third method is to calculate the average atomic mass. This is done by taking the mass of all the naturally occurring isotopes of an element and dividing it by the total number of isotopes. For example, the average atomic mass of chlorine is 35.45 amu.
Importance of Atomic Mass
Atomic mass is an important property for understanding the physical and chemical properties of an element. It is used to calculate the atomic weight of an element, which is the average mass of the element’s atoms. Atomic weight is often used to determine the amount of a certain element in a compound or mixture. It is also used to calculate the molar mass of a substance, which is the mass of one mole of a substance.
Atomic mass is also important for understanding the behavior of atoms. It is used to calculate the binding energy of an atom, which is the energy required to break the atom apart. It is also used to calculate the average kinetic energy of an atom, which is the energy that an atom has due to its motion.
Atomic mass is an essential property of all atoms. It is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass in a group of atoms. Atomic mass is typically expressed in atomic mass units (amu). It is used to calculate the atomic weight of an element and to determine the amount of a certain element in a compound or mixture. Atomic mass is also important for understanding the behavior of atoms, as it is used to calculate the binding energy and the average kinetic energy of an atom.
How do you find the mass of an atom?
Atoms are the building blocks of all matter, and understanding their mass is essential to understanding chemistry and physics. But how do you find the mass of an atom?
The answer depends on the type of atom you’re looking at, as well as the information you’re given. In this article, we’ll look at three ways to find atomic mass: by looking it up on the periodic table, calculating the averaged atomic weight of an element, and calculating the relative atomic mass of a sample.
1. Look Up Atomic Mass on the Periodic Table
If you’re just starting out in chemistry, your instructor will likely want you to use the periodic table to find the atomic mass (or atomic weight) of an element. The periodic table is an organized chart that shows the atomic number, symbol, and mass of each element.
The atomic mass is usually expressed in atomic mass units (amu), also known as daltons. One atomic mass unit is equal to one-twelfth of the mass of an atom of carbon-12.
2. Calculate the Averaged Atomic Weight of an Element
The atomic mass of an element is an average based on the natural abundance of its isotopes. Isotopes are atoms of the same element that have different numbers of neutrons.
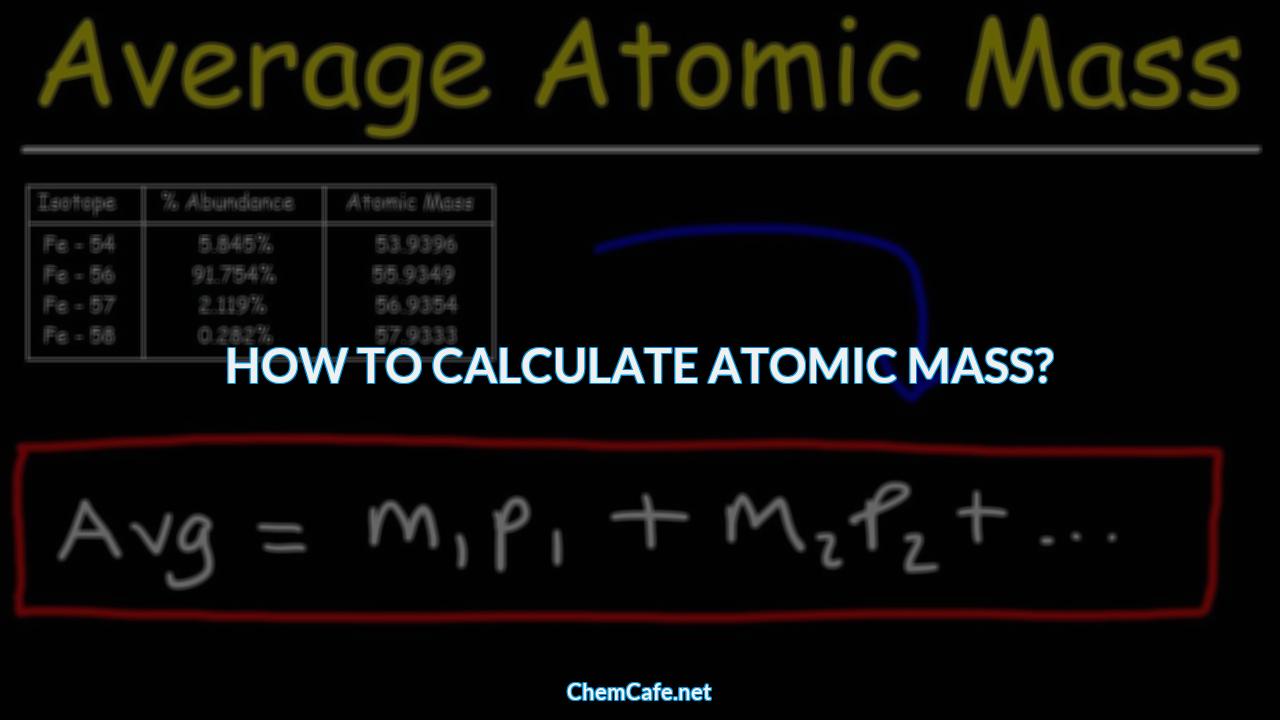
To calculate the averaged atomic weight of an element, you need to know the relative atomic masses of its isotopes, as well as their natural abundance. Then, you can use the following formula:
Averaged Atomic Weight = (Relative Atomic Mass of Isotope 1 x Natural Abundance of Isotope 1) + (Relative Atomic Mass of Isotope 2 x Natural Abundance of Isotope 2) + …
3. Calculate the Relative Atomic Mass of a Sample
If you’re dealing with a sample of atoms that contains a known ratio of isotopes, you can calculate the relative atomic mass of the sample. This can be done using the following formula:
Relative Atomic Mass = (Relative Atomic Mass of Isotope 1 x Number of Atoms of Isotope 1) + (Relative Atomic Mass of Isotope 2 x Number of Atoms of Isotope 2) + …
Conclusion
The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and electrons determines its charge. Each atom of an element contains the same number of protons, known as the atomic number (Z).
Depending on the type of atom you’re looking at, you can find its mass by looking it up on the periodic table, calculating the averaged atomic weight of an element, or calculating the relative atomic mass of a sample. With a basic understanding of atoms and isotopes, you’ll be able to find the mass of an atom in no time.
How is atomic mass calculated?
Atomic mass is a fundamental property of the atoms that make up matter and can be used to identify and classify elements. Knowing how to calculate atomic mass is important for studying chemistry and physics. In this article, we’ll discuss what atomic mass is, how it is calculated, and the three different methods of calculating it.
What Is Atomic Mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. It is measured in atomic mass units (amu). One atomic mass unit is equal to one-twelfth of the mass of an atom of carbon-12.
Adding the Masses of Protons and Neutrons
In order to calculate the mass of a single atom of an element, you can consider using the number of the protons and neutrons present in its nucleus. By adding the masses of each proton and neutron present in the nucleus of a given atom, you can extract its atomic mass. In the vague terminology, atomic mass can also be defined as the number of the protons and neutrons.
Three Different Methods to Calculate the Atomic Mass
There are three different methods to calculate the atomic mass:
- By having reference to the Periodic Table – In the periodic table, an atomic number is typically indicated under the representation of an element. For example, the atomic number of carbon is 6, as seen in the image below.
(Image will be Uploaded Soon)
In general, however, the atomic mass of an atom will be very similar to its mass number, although the decimal places will have some variation. - By using the Relative Atomic Mass – The relative atomic mass (RAM) is an average of the masses of all the isotopes of an element. This method is useful when the exact number of protons, neutrons, and electrons in an atom is unknown.
- By calculating the Mass Defect – The mass defect is the difference between the mass of a nucleus and the sum of its individual nucleons. This method is useful when the masses of the individual protons and neutrons are known.
Atomic mass is an important property of atoms and can be calculated using three different methods. Depending on the information you’re given, you can use the periodic table, relative atomic mass, or mass defect to calculate the atomic mass of an element.
How do you find the atomic number and mass?
Atoms are composed of protons, neutrons, and electrons, and each of them has a distinct role in an atom’s structure. The number of protons, neutrons, and electrons determine the atomic number and mass number of an atom. Knowing the atomic number and mass of an atom can help you understand the properties of an element and can be useful in chemistry calculations. Let’s take a look at how to find the atomic number and mass.
Name, Location, Charge, and Mass of Subatomic Particles
The three main subatomic particles are protons, neutrons, and electrons. Protons are positively charged particles located in the nucleus of an atom, with a mass of 1 atomic mass unit (amu). Neutrons are neutral particles located in the nucleus of an atom, with a mass of 1 amu. Electrons are negatively charged particles that orbit the nucleus of an atom, with a mass of 0 amu.
Finding the Atomic Number
The atomic number of an element is the number of protons in its nucleus. This number is the same for all atoms of the same element, and it is the number used to identify elements on the periodic table. To find the atomic number, you can simply look up the element on the periodic table. Elements are arranged in increasing atomic number from left to right and top to bottom, so you can easily locate the element and its corresponding atomic number.
Atomic Number vs. Mass Number
Atomic number and mass number are related but different. The atomic number is the number of protons in an atom’s nucleus, whereas the mass number is the sum of the number of protons and neutrons in the nucleus. Knowing the atomic number and mass number of an atom can help you calculate the number of neutrons in the atom’s nucleus.
The mass number of an atom is the sum of the number of protons and neutrons in the atom’s nucleus. It is reported in atomic mass units (amu). For example, the mass number of carbon-12 is 12 amu, which is the sum of its 6 protons and 6 neutrons.
Atomic number and mass number are useful in understanding the structure of an atom and in chemistry calculations. The atomic number is the number of protons in an atom’s nucleus, and it can be easily found on the periodic table. The mass number is the sum of the number of protons and neutrons in the nucleus and is reported in atomic mass units (amu). Knowing the atomic number and mass of an atom can help you understand the properties of an element and can be useful in chemistry calculations.


Leave a Comment