Why Are Atomic Mass and the Mass Number of an Isotope Equivalent?
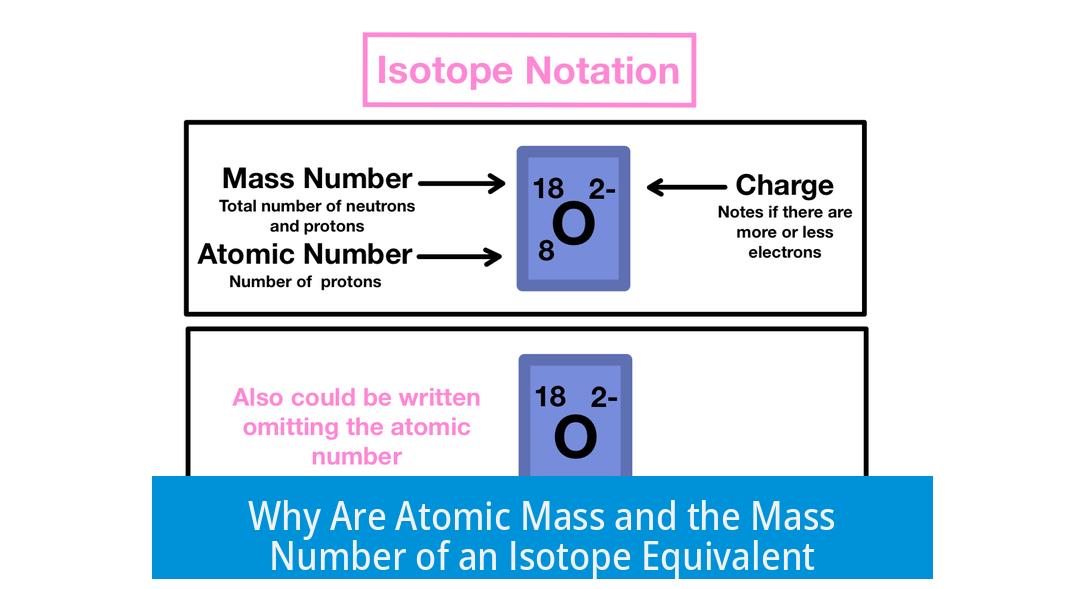
The mass number of an isotope and its atomic mass are nearly equivalent because both represent the total count and approximate total mass of protons and neutrons in that specific isotope’s nucleus. However, the atomic mass includes small variations due to the exact masses of these particles, while the mass number is a simple whole count.
Definitions and Basic Concepts
The mass number of an isotope is the total number of protons and neutrons in its nucleus. It is a whole number and serves as a specific count for that atom.
The atomic mass is measured in atomic mass units (amu). One amu corresponds roughly to the mass of one proton or one neutron.
Relationship Between Mass Number and Atomic Mass
For a specific isotope, the mass number is the sum of protons and neutrons. The atomic mass for that isotope closely corresponds to this sum because protons and neutrons have nearly the same mass.
However, the masses of protons (approximately 1.0073 amu) and neutrons (about 1.0087 amu) differ slightly, so atomic mass is not exactly equal to the mass number but very close.
Atomic Mass as a Weighted Average vs. Mass Number as a Specific Count
The atomic mass listed for an element on the periodic table is a weighted average of all its naturally occurring isotopes, which vary in neutron number and abundance.
- For example, hydrogen’s atomic mass is around 1.008 amu, not exactly 1, because it is mostly protium (mass number 1) but also includes about 0.015% deuterium (mass number 2).
- Chlorine’s atomic mass (~35.45 amu) reflects the mix of its two major isotopes, chlorine-35 and chlorine-37, weighted by their natural abundances.
Therefore, the mass number applies to one specific isotope, representing an exact count of nucleons, while the atomic mass often reflects an average across isotopes, causing slight differences.
Key Takeaways
- Mass number is the whole number count of protons plus neutrons in one isotope.
- Atomic mass is the mass of an isotope in atomic mass units, approximating mass number but accounting for particle mass differences.
- Atomic mass on the periodic table is typically a weighted average of all isotopes of an element.
- For a single isotope, atomic mass and mass number are nearly equal but not exactly the same.
- Differences arise because protons and neutrons have slightly different masses, and neutron-proton binding energy affects precise atomic mass.
Why Are Atomic Mass and the Mass Number of an Isotope Equivalent?
At first glance, atomic mass and the mass number of an isotope seem like two sides of the same coin. That’s because, in many cases, their values are almost identical—but why? The short answer: both relate to the protons and neutrons in the nucleus. But the details reveal a fascinating mix of precision, averages, and the quirks of subatomic particles.
Let’s unpack this together, so you can confidently explain to your friends (or at least impress them) why these two concepts often line up so nicely.
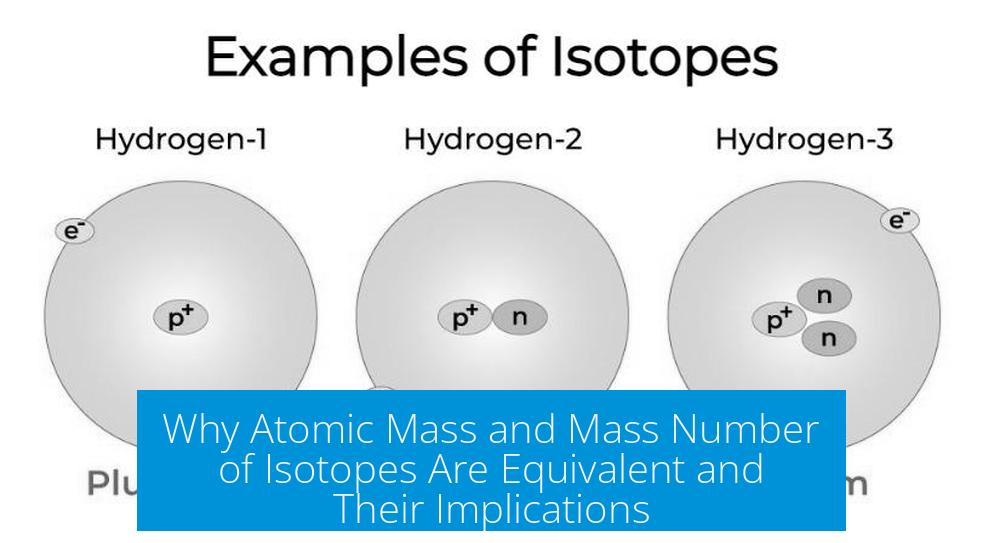
Starting With the Basics: What’s the Mass Number of an Isotope?
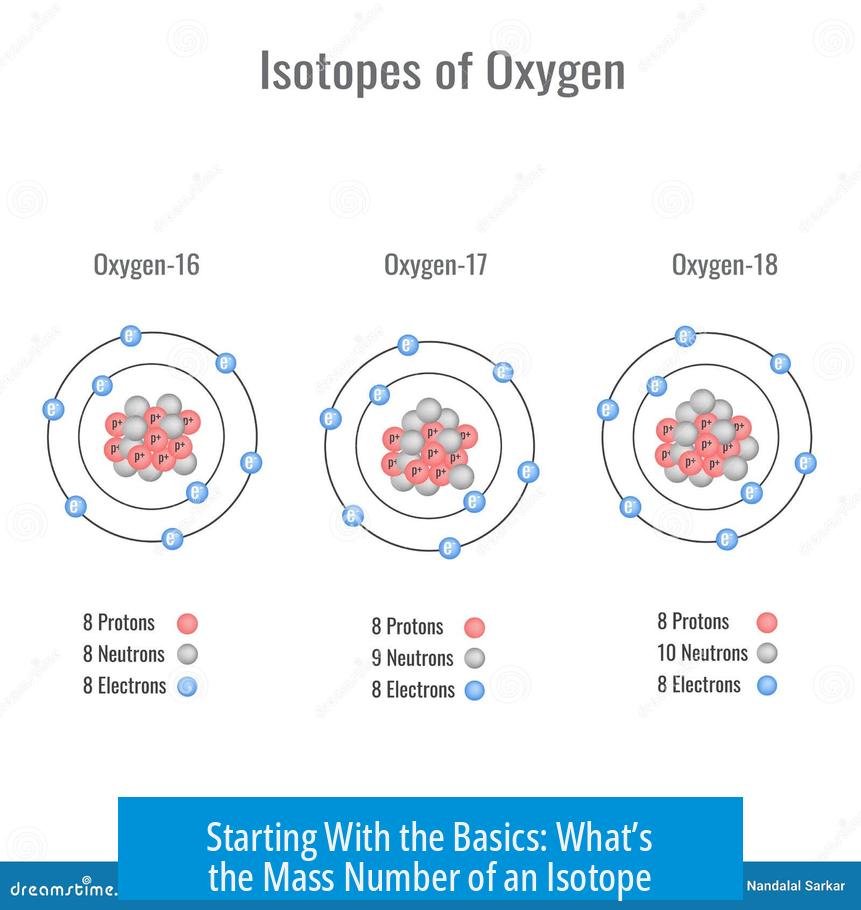
The mass number, in simple terms, is just a count – an exact count – of all the protons and neutrons packed inside the nucleus of a specific atom of an isotope. If you imagine the nucleus as a tiny billiard ball cluster, the mass number tells you the total balls there.
Why protons and neutrons? Because electrons weigh almost nothing by comparison.
- Protons: Positively charged particles, defining the element.
- Neutrons: Neutral particles, varying between isotopes.
So, for example, carbon-12’s mass number is 12, meaning 6 protons and 6 neutrons. Pretty straightforward, right?
But What About Atomic Mass? Isn’t It Just the Same?
Almost, but not quite. Atomic mass is a little bit more like a math teacher who likes to average everyone’s grades. It is measured in atomic mass units (amu), where 1 amu approximates the mass of a proton or neutron.
But unlike the mass number’s exact count of particles, atomic mass is a weighted average of all the isotopes’ masses of an element that occur naturally. This means atomic mass combines the masses of various isotopes but accounts for how common each one is. It’s like mixing different flavors of ice cream and giving more weight to your favorite flavor.
Why Are They Often Equivalent Then?
Great question. The trick is that many elements have a “star player” isotope — a dominant isotope that makes up the majority of the naturally occurring sample.
For these elements, their average atomic mass inches very close to the mass number of this main isotope. For example, hydrogen’s average atomic mass is approximately 1.00794 amu, which is close to the mass number of its most common isotope (1).
This is because most of the hydrogen atoms out there have just one proton and zero neutrons, making their mass number 1, which closely aligns with the average atomic mass.
But—plot twist!—Why Aren’t They Exactly the Same?

Now let’s peek behind the curtain. First, protons and neutrons don’t weigh exactly the same. A neutron is just a smidge heavier than a proton. So, adding them up won’t always give the perfectly neat number you’d expect.
Second, the atomic mass you see on the periodic table is not of a single isotope but an average of all the isotopes mixed in. Some elements, like chlorine, have two very stable isotopes with different masses, so the average falls somewhere in between.
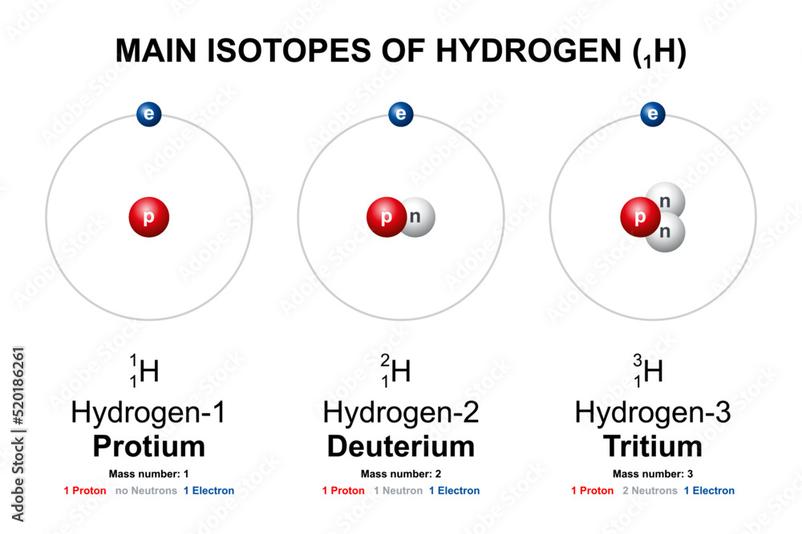
Take hydrogen’s isotopes for example:
- Protium (most common): 1 proton, 0 neutrons, mass number 1.
- Deuterium: 1 proton, 1 neutron, mass number 2.
- Tritium: 1 proton, 2 neutrons, mass number 3 (rare, radioactive).
Because a small percentage of hydrogen atoms exist as deuterium, the overall average atomic mass bumps just above 1, landing at 1.00794 on the periodic table.
What Does This Mean for You?
Understanding this subtle difference helps demystify the numbers in your chemistry textbooks and periodic tables. It also clarifies why some elements don’t have a snug mass number equal to atomic mass.
If you’re diving into scientific work or just passing exams, remember these key points:
- Mass Number: Exact count of protons and neutrons in a specific isotope’s nucleus.
- Atomic Mass: Weighted average of all isotopes’ masses for an element, in amu.
- Many elements’ atomic masses approximate the mass number of their most common isotope.
- The tiny differences in neutron/proton masses and isotope abundances cause slight mismatches.
Does This Affect Chemical Behavior?
Interestingly, isotopes (atoms with different mass numbers but same protons) behave very similarly chemically. This is because chemistry mostly involves electrons, not the nucleus.
However, in fields like nuclear medicine or radiometric dating, knowing the precise isotope and its mass is vital.
A Quick Table Example: Carbon Isotopes
| Isotope | Protons | Neutrons | Mass Number | Atomic Mass (amu) | Natural Abundance (%) |
|---|---|---|---|---|---|
| Carbon-12 | 6 | 6 | 12 | 12.0000 | 98.9 |
| Carbon-13 | 6 | 7 | 13 | 13.0034 | 1.1 |
The average atomic mass of carbon on the periodic table is about 12.01 amu — closer to carbon-12’s mass number, reflecting its dominance in abundance.
Wrapping It Up With a Fun Twist
So, next time you see atomic mass and mass number lurking around the periodic table, you’ll know they’re like siblings who look a lot alike but each have their own quirks.
Mass number is the precise headcount of protons plus neutrons in one specific isotope. Atomic mass is the average weight of all the isotope siblings weighed by how popular each is in nature.
Knowing this adds a punch of depth to your chemistry knowledge. You’ll understand why atomic mass values seem close but sometimes prefer to keep their distance. It’s all in the numbers, the particles, and a pinch of isotope diversity.
Next question: Could those tiny mass differences lead to cool tech breakthroughs like isotope-based medical treatments or cleaner energy? That’s a story for another post!
What makes the atomic mass and mass number nearly equal for one isotope?
The mass number counts protons and neutrons in one atom. Atomic mass is based on proton and neutron masses, so for one isotope, both values closely match.
Why is atomic mass not exactly the same as mass number?
Protons and neutrons do not have exactly equal masses. Also, atomic mass is a weighted average of all isotopes, while mass number is just a count for one isotope.
How does isotope abundance affect atomic mass?
The atomic mass reflects the weighted average of all natural isotopes. If one isotope is more common, the atomic mass will be closer to its mass number.
Can atomic mass represent multiple isotopes of an element?
Yes, atomic mass averages the masses of all isotopes. This average can differ from the mass number of any single isotope.
Why does hydrogen have an atomic mass of 1.00794 instead of exactly 1?
Hydrogen’s atomic mass is slightly above 1 because of the presence of deuterium, an isotope with one neutron, which raises the average mass value.




Leave a Comment