Why Are the Lanthanide and Actinide Series Separated in the Periodic Table?
The lanthanide and actinide series are separated from the main body of the periodic table mainly to reduce the table’s width for practical reasons, while their position corresponds to their unique outermost f electron configurations.
Practicality of Table Width
The periodic table’s design aims to maintain readability and fit on standard pages or posters. If the lanthanides and actinides were included in the main table, it would become extremely wide—adding 14 elements for each series. This width would be inconvenient for display.
Separating these two series shortens the table by roughly three columns, making the chart more compact and manageable. This separation is a practical choice rather than a scientific necessity. The f-block elements could be placed without separation, but the overall presentation would suffer.
Electron Configuration and the f-Block
The periodic table organizes elements based on their valence electrons. The lanthanides and actinides belong to the f-block, as their outermost electrons occupy f orbitals. There are 7 f orbitals that can hold up to 14 electrons, matching the 14 elements of each series.
Other blocks follow this pattern based on orbitals:
- s-block: 2 columns
- p-block: 6 columns
- d-block: 10 columns
- f-block: 14 columns
The lanthanides and actinides fill these f orbitals, distinguishing them from s, p, and d block elements.
Historical and Visual Presentation Factors
Historically, the lanthanides and actinides appeared as extensions of the table to maintain clarity. Several versions of the periodic table show the f-block embedded in the main table, but these are often too wide for practical use.
Splitting the f-block from the main table is a convention aimed at easier visualization and efficient use of space in textbooks and other educational materials. This convention persists despite no strict scientific rule demanding it.
Key Takeaways
- The lanthanide and actinide separation reduces the periodic table’s width for easier display.
- These series correspond to f-block elements with outermost electrons in f orbitals.
- Historical conventions and presentation needs influence this layout choice.
- The separation is practical rather than scientifically mandated.
Why Are the Lanthanide and Actinide Series Separated in the Periodic Table?
The lanthanide and actinide series are separated mainly to reduce the width of the periodic table for practical drawing and presentation purposes. That’s the core reason that most chemistry teachers and textbooks stick to. But let’s dive deeper because this simple answer barely scratches the surface.
Have you ever tried fitting a huge, ultra-detailed periodic table onto a poster or a book page? It’s like trying to squeeze a giraffe into a Mini Cooper—awkward and impractical. The lanthanides and actinides are special groups of elements that add a whopping 14 columns each when placed inline with the main body of the table. You’d end up with a periodic table that’s impractically wide, sprawling across your wall or computer screen in an unwieldy fashion.
Separating these two series—the lanthanides and the actinides—into their own rows below the main table saves a ton of space. This keeps the periodic table manageable, both for printing and for everyday use. Without this adjustment, periodic tables would extend horizontally like an endless parking lot rather than a neatly organized chart. It’s more about practical presentation than chemistry being stubborn or secretive about these element sets.
Electron Configuration: The Real Science Behind Their Special Status
Sure, convenience is one answer, but the lanthanides and actinides also occupy a unique spot because of their electronic structure. The periodic table groups elements based on their valence electrons—the outermost electrons that determine chemical behavior. You have different blocks: s, p, d, and then f.
- The left two columns correspond to the s-block, where elements have outer electrons in s orbitals.
- The central 10 columns are the d-block, also known as transition metals, whose valence electrons live in d orbitals.
- Then you have the right side, the p-block, with electrons in p orbitals.
- Last but certainly not least come the f-block elements, where electrons occupy those lesser-known but super interesting f orbitals.
F orbitals are a bit like the hidden backstage crew in the atomic theater—there are 7 orbitals in this category, each capable of holding 2 electrons. So, that’s 14 potential electrons in total for each f-block row. The lanthanides fill once the 4f orbitals start to fill, and the actinides are where the 5f orbitals take center stage.
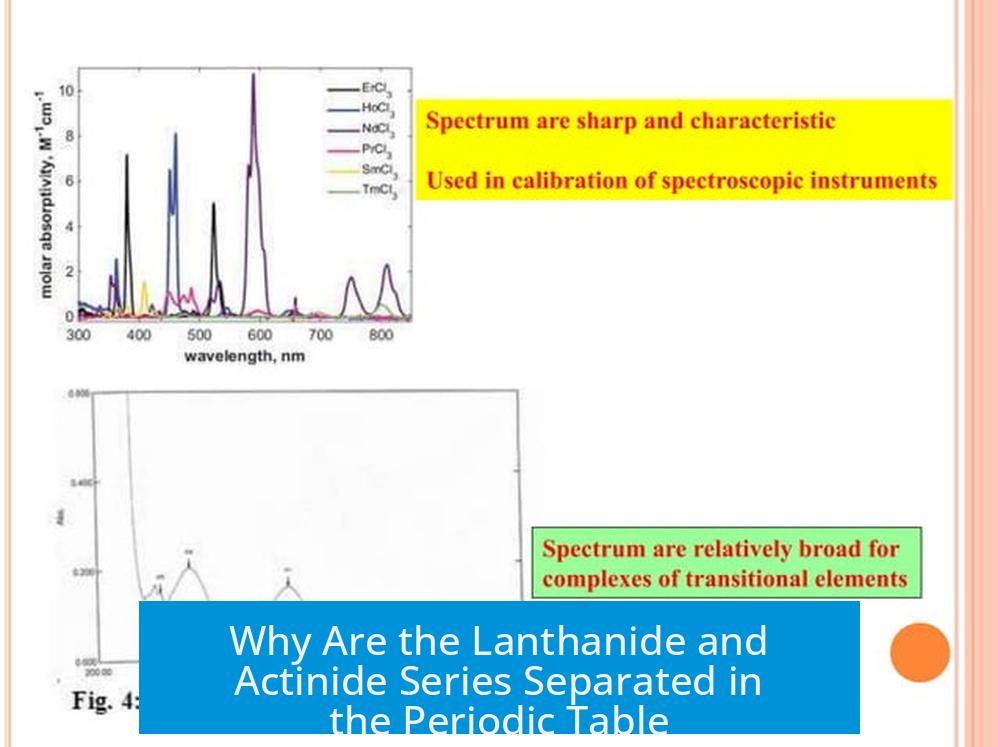
Because of this electron configuration, the lanthanides and actinides belong to the f-block categories, distinct in their chemistry and physics from the s, p, and d blocks. Their chemical properties, reactivity, and even colors are heavily influenced by these f electrons, which makes these series particularly interesting to chemists and physicists alike.
Historical Context: It Wasn’t Always This Way
Believe it or not, our current periodic table layout is as much a product of history and compromise as it is of science. When scientists first started placing elements in order, the lanthanides and actinides were a bit of a headache. The long tables with all f-block elements positioned “properly” in line created what’s called a “32-column” periodic table.
This version, while scientifically accurate, was cumbersome. Imagine trying to sketch a periodic table on a textbook page or a classroom whiteboard with 32 columns. It quickly became clear that a compromise was needed—one that retained scientific meaning but improved usability.
Now, most periodic tables you see have the lanthanides and actinides as two separate rows at the bottom. This arrangement became standard because it simplified the overall design and made the table easier to read and teach without losing the valuable information on valence electrons and atomic numbers.
Could We Have a Continuous Table Without Separation?
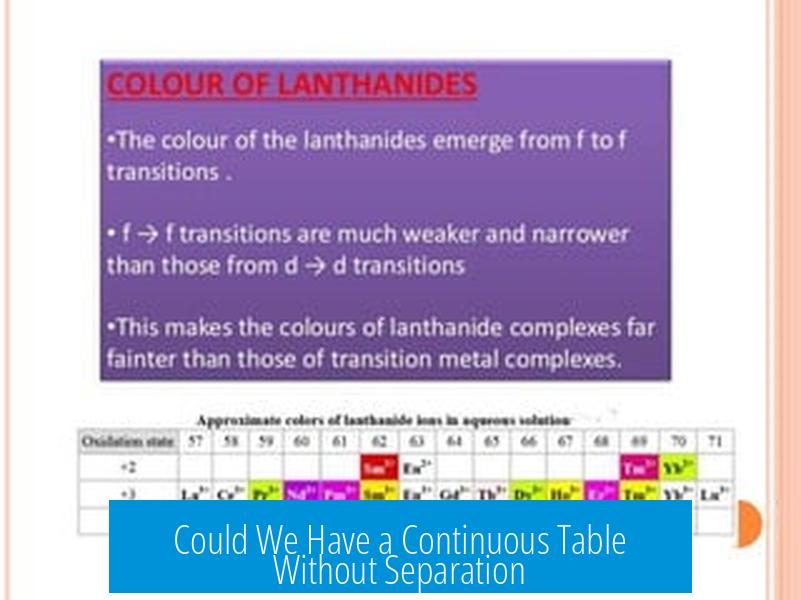
Absolutely! Several “long form” periodic tables place these f-block elements in their “correct spots” without separating them. But then, they become challenging to fit on traditional media and can intimidate students or novices due to their sheer width.
Even though such full-width tables exist online or in large scientific publications for detail-oriented work, everyday use favors the separated layout. So, the question becomes: what do you prioritize? Scientific exactness or practical display? The normalized periodic table opts for the latter, balancing clarity and information.
What Does This Mean for You?
If you’re a student wondering why your periodic table has those two rows hanging out at the bottom, now you know it’s not some arbitrary quirk. It’s all about making the vast landscape of elements fit on a page without losing the essential structure that reflects their chemical nature.
When you see the lanthanides and actinides, think of them as the special backstage performers in the elemental drama. Their placement in the periodic table invites you to appreciate their unique properties while keeping the whole show manageable for viewers.
So next time you glance at a periodic table, give a nod to those separated rows. They’re doing more than just saving space—they’re preserving clarity and helping everyone understand the amazing world of chemistry.
Quick Recap:
- Width reduction: Separating the lanthanides and actinides keeps the periodic table compact and practical.
- Electron configuration: Their f-block electron arrangement differentiates them from other blocks.
- Historical reasons: Early long tables were too wide; separation became a teaching and usability standard.
- Practicality over perfection: Full-width tables exist but aren’t user-friendly for everyday learning and use.
A Fun Thought
Imagine if the periodic table was printed on your socks—do you want those f-blocks to stretch across your ankles or be neatly folded under, like a quirky cuff? Presentation matters, folks!
“Good design is as much about what you leave out as what you put in,” said someone who probably would have loved the idea of separating the f-block.
Why are the lanthanide and actinide series shown separately from the main periodic table?
They are separated mainly to keep the table from becoming too wide. This arrangement makes it easier to fit the periodic table on standard pages and posters.
How does electron configuration relate to the placement of lanthanides and actinides?
Lanthanides and actinides have outermost electrons in the f orbitals. This f-block classification is different from s, p, and d blocks, justifying their unique location on the table.
Could the periodic table include lanthanides and actinides without separation?
Yes, but it would make the table very wide and hard to display. Keeping them separate shortens the table’s width by several columns and improves readability.
Are there scientific reasons for separating these two series apart from convenience?
Fundamentally, no. The main reasons are historical and practical for space and presentation. Their electron configuration supports their block grouping but not the physical separation.
Is there a version of the periodic table where lanthanides and actinides are not separated?
Yes, some extended periodic tables show the f-block elements in their full position without separation. However, these versions are wider and less common in everyday use.





Leave a Comment