Why Does Acetone Evaporate From the Cuvette So Quickly?

Acetone evaporates quickly from the cuvette because its physical properties favor rapid vaporization, and mechanical disturbances disrupt the vapor layer above the liquid, enhancing evaporation rates. The combination of acetone’s high vapor pressure, weak intermolecular forces, and low enthalpy of vaporization means it requires less energy to enter the gas phase. Physical actions like tapping or moving the cuvette disturb the vapor-air interface. This disturbance prevents the buildup of saturated acetone vapor near the liquid surface, allowing more molecules to escape into the air and increasing the evaporation rate.
1. Evaporation Dynamics and the Role of Vapor Layer

When acetone is left stationary inside a cuvette, it begins to evaporate, forming a thin layer of acetone vapor above its liquid surface. This vapor layer behaves with laminar flow and creates a concentration gradient—the vapor concentration is highest near the liquid and decreases with distance into the air.
This gradient slows evaporation because fewer acetone molecules can escape once the air directly above becomes saturated. The system reaches a dynamic equilibrium where evaporation and condensation rates balance out.
However, when the cuvette moves—such as tapping, shaking, or waving—the vapor layer breaks down. Movement mixes the acetone vapor with ambient air, lowering the local vapor concentration near the surface. This disrupted gradient promotes faster evaporation by allowing more acetone molecules to escape before saturation stalls the process.
2. Effects of Mechanical Disturbance on Evaporation
Mechanical disturbance of the cuvette accelerates acetone evaporation in multiple ways:
- Disturbing the Diffusive Layer: By shaking or tapping the cuvette, the diffusive boundary layer between the liquid and air breaks down. This increases the flux of vapor molecules from the liquid to the air.
- Increasing Surface Area: Tapping can spread acetone across the cuvette walls in a thin film. This larger surface area exposes more molecules to the air, accelerating evaporation.
- Mixing Vapor With Air: Movement causes the acetone vapor to mix with surrounding air rapidly. It prevents the local vapor concentration from reaching saturation.
These factors mirror blowing over a liquid surface to hasten evaporation but occur here naturally from simple cuvette handling.
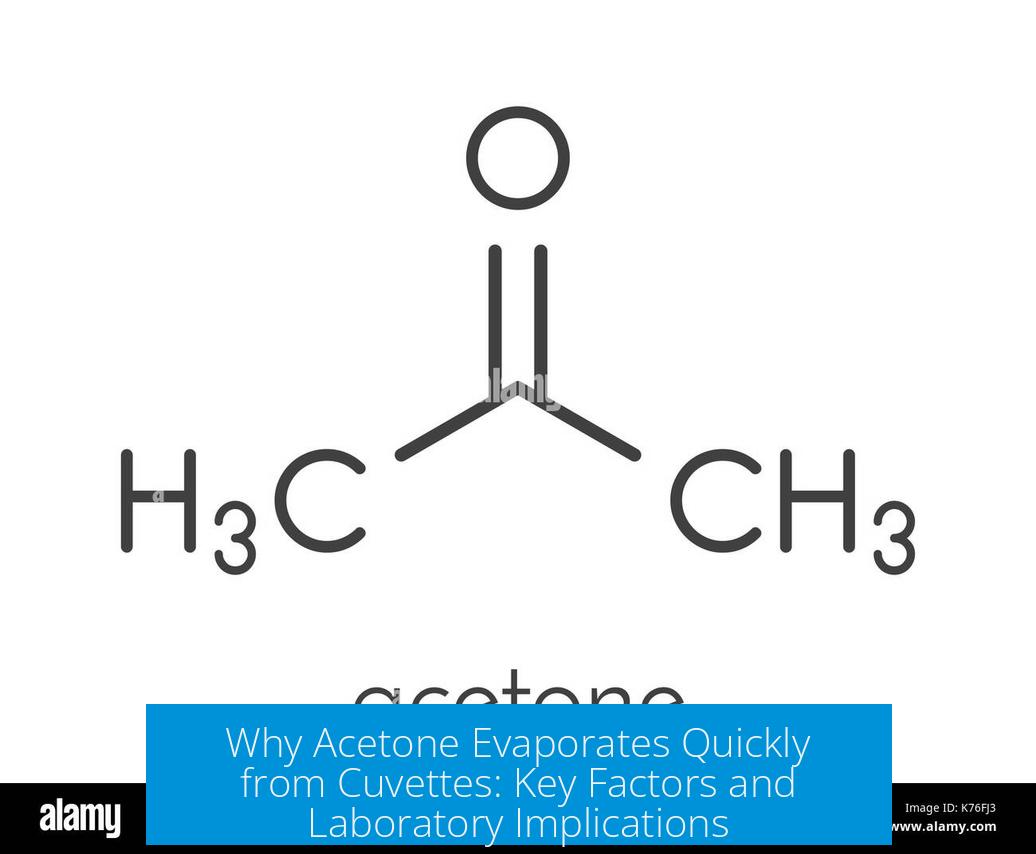
3. Acetone’s Chemical and Physical Properties Leading to Rapid Evaporation
Acetone’s molecular properties are critical to its fast evaporation:
| Property | Value or Description | Effect on Evaporation |
|---|---|---|
| Vapor Pressure | ~30 kPa at 20 °C | High vapor pressure encourages molecules to escape liquid rapidly. |
| Enthalpy of Vaporization | 31.3 kJ/mol | Low energy required to vaporize compared to similar solvents. |
| Intermolecular Forces | Weak dipole-dipole; no strong hydrogen bonding | Easier bond breaking, facilitating quick evaporation. |
| Boiling Point | 56 °C | Relatively low boiling point increases volatility at room temperature. |
The weak intermolecular forces reduce the energy acetone molecules need to overcome to transition from liquid to gas. This contrasts with water, which has strong hydrogen bonds and a much higher enthalpy of vaporization, making water evaporate slower under similar conditions.
4. Vapor Density and Orientation Effects
Acetone vapor is denser than the surrounding air. When the cuvette is held upright, the vapor tends to pool over the liquid surface. This pooling forms a barrier to evaporation by increasing the local vapor concentration directly above the acetone.
Tilting the cuvette or orienting it upside down mixes the denser acetone vapor with air differently. The vapor layer breaks and convection currents more easily disperse acetone molecules. This effect further increases evaporation by preventing vapor saturation near the liquid.
5. Air Saturation and the Influence of Airflow
The rate of evaporation depends on the relative saturation of acetone vapor in the surrounding air.
- If the air near the liquid becomes saturated with acetone vapor, evaporation slows down because the concentration gradient driving vaporization decreases.
- If airflow or movement removes vapor-saturated air and replaces it with cleaner air, evaporation speeds up.
In practical terms, acetone in a sealed or “flipped” cuvette saturates the trapped air quickly. This saturation limits further loss by evaporation. However, if airflow or movement constantly refreshes the air around the cuvette, acetone evaporates continuously and rapidly.
Mechanical disturbance mimics this by moving vapor away, reducing local acetone vapor pressure near the liquid surface.
6. Minor Contribution of Heat From Handling
Though humidity or temperature can affect evaporation, heat from touching the cuvette likely plays a minor role. The body heat transferred during tapping could increase the liquid temperature marginally, but this effect is negligible compared to mechanical and physical factors.
Evaporation primarily depends on vapor pressure, airflow, and surface dynamics rather than slight temperature changes from hand contact.
7. Relevance in Laboratory Settings
In analytical chemistry labs, rapid evaporation of acetone in cuvettes improves efficiency. Sample preparation often requires drying solvent residues to proceed with measurements.
Mechanical tapping or moving a cuvette containing acetone reduces drying times significantly—often completing evaporation in 3 seconds instead of 10 or more while stationary. This speed enhances throughput when processing many samples daily.
Understanding these factors can help lab technicians optimize protocols, increase sample processing speed, and maintain accuracy in versatile workflows.
Summary of Key Points
- Acetone’s physical properties—high vapor pressure, low enthalpy of vaporization, and weak molecular bonds—make it evaporate rapidly.
- Stationary evaporation forms a vapor layer that slows evaporation via a concentration gradient.
- Mechanical disturbance disrupts the vapor layer, spreads acetone on cuvette walls increasing surface area, and mixes vapor with air, all speeding evaporation.
- Acetone vapor density can cause vapor pooling; changing cuvette orientation and convection breaks this layer, enhancing evaporation.
- Air saturation affects evaporation rates; airflow refreshes air, preventing vapor buildup and maintaining fast evaporation.
- Heat transfer from handling has minimal impact compared to physical disturbance and acetone’s properties.
- Fast evaporation is valuable in labs for quick sample drying and efficient workflows.
Why does acetone evaporate faster when the cuvette is moved or tapped?
Moving or tapping the cuvette disturbs the vapor layer above the acetone. This mixes acetone vapor with air and lowers its local concentration, speeding up evaporation.
How does spreading acetone on cuvette walls affect evaporation?
Spreading acetone creates a thin film with a larger surface area. More surface means more molecules can escape, causing faster evaporation.
What role do acetone’s physical properties play in its quick evaporation?
Acetone has weak molecular bonds and a high vapor pressure. These cause it to vaporize with less energy, making it evaporate faster than many solvents.
Does the orientation of the cuvette affect acetone evaporation? How?
Yes. Acetone vapor is denser than air and pools above the liquid when upright, slowing evaporation. Changing orientation or enabling air movement breaks this layer and accelerates evaporation.
Why does airflow around the cuvette influence acetone’s evaporation rate?
Airflow removes saturated acetone vapor near the liquid. This prevents vapor buildup, allowing more acetone molecules to vaporize, thus increasing evaporation speed.
Can heat from handling the cuvette significantly speed up acetone evaporation?
Heat from hands has a minor effect. Mechanical disturbance and acetone’s inherent properties mainly drive the fast evaporation.



Leave a Comment