Why Does an Atom Become Positively Charged When It Shares Its Lone Pair?
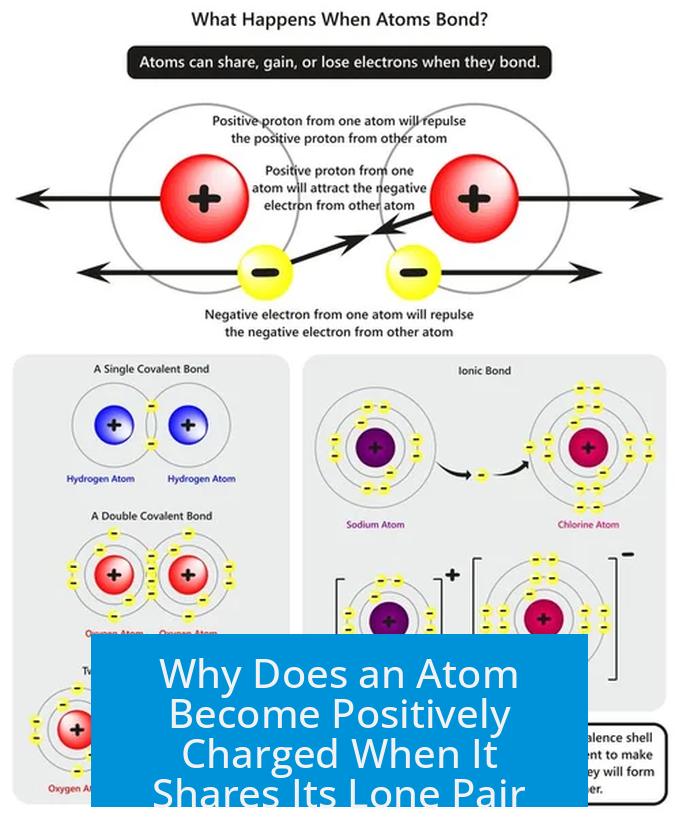
An atom becomes positively charged when it shares its lone pair because it effectively loses control over one of its electrons during bond formation. This results in a reduced electron count around the atom compared to its proton count, creating a positive formal charge.
Understanding Electron Sharing in Covalent Bonds
Covalent bonds arise from the sharing of electron pairs between atoms. Each atom involved typically “owns” one electron from the shared pair, with the electrons considered divided between the two nuclei. For example, when nitrogen in ammonia (NH3) donates its lone pair to bond with a hydrogen ion (H+), it forms a new covalent bond. This bond features one electron attributed to nitrogen and one considered “belonging” to hydrogen.
In this case, nitrogen starts with a lone pair—two electrons localized solely on it. When these electrons are shared to bond with H+, nitrogen no longer has exclusive access to both. As a result, it effectively “loses” one electron to hydrogen. The hydrogen ion gains that electron, becoming neutral hydrogen, while nitrogen acquires a deficit of electron density.
Electron Distribution in Covalent Bonds
Within a covalent bond, the electrons are evenly distributed in terms of time spent near each nucleus. At any moment, one electron might be closer to one atom and the other electron closer to the other. Over time, the bond averages out to one electron per atom.
Prior to bonding, a lone pair is fully localized on one atom, contributing fully to its electron count and charge balance. When that lone pair forms a bond, the electron density splits, and the atom shares the pair rather than solely possessing it.
How Nitrogen’s Electron Count Affects Its Charge
Nitrogen, with 7 protons, needs 7 electrons to balance its positive nuclear charge and remain neutral. It has 5 valence electrons in total, typically possessing 3 bonding pairs and one lone pair in ammonia (NH3).
In NH3, nitrogen forms three bonds with hydrogen atoms and holds onto a lone pair. Its formal charge calculation equals zero because nitrogen effectively “owns” all 5 valence electrons:
- 5 valence electrons (VE)
- Minus 3 bonds (each counts as one electron shared)
- Minus 2 electrons in lone pairs (LP)
The calculation: 5 VE – 3 (bonds) – 2 (LP) = 0 charge.
However, in the ammonium ion (NH4+), nitrogen forms four bonds and does not maintain any lone pairs. Now, nitrogen effectively “owns” fewer electrons since all are shared in bonding:
- 5 valence electrons
- Minus 4 bonds
- Minus 0 lone pairs
Calculation: 5 VE – 4 (bonds) – 0 (LP) = +1 formal charge.
The formal charge of +1 arises because nitrogen now “counts” only four electrons (from bonding) but still has seven protons in its nucleus. Thus, nitrogen carries a positive charge, reflecting the electron deficit around it.
Formal Charge and Its Role in Charge Assignment
The notion of formal charge helps quantify changes in electron ownership during bonding. It is calculated as:
Formal Charge = (Valence Electrons) – (Number of Bonds) – (Electrons in Lone Pairs)
This formula treats bonding electrons as shared equally. The lone electrons are fully assigned to the atom. When an atom donates its lone pair to form a bond, the count of lone pair electrons drops, while the number of bonds rises.
For nitrogen:
| Molecule | Valence Electrons (VE) | Number of Bonds | Lone Pair Electrons (LP) | Formal Charge |
|---|---|---|---|---|
| NH3 | 5 | 3 | 2 | 0 (neutral) |
| NH4+ | 5 | 4 | 0 | +1 (positive) |
The Role of Lone Pairs in Charge Distribution
Lone pairs carry negative charge because electrons are negatively charged. When an atom donates its lone pair to form a bond, it reduces the localized negative charge on that atom.
Effectively, this reduces the electron density around the atom, which can be understood as the atom losing some negative charge. Since the protons remain unchanged in the nucleus, this imbalance manifests as a positive charge.
In bonding, lone pairs act as electron donors. Sharing them alters the electron distribution. The atom giving up part of its lone pair partially loses electron density and picks up a positive formal charge.
Practical Implications in Chemistry
This concept explains why certain molecules or ions carry a positive charge after forming coordinate covalent bonds using lone pairs. Ligands donating lone pairs to metal centers, or nitrogen in ammonium ion formation, follow this principle.
Understanding the charge changes informs interpretations of molecular structure, reactivity, and stability. Chemists use formal charges to predict which atoms in a molecule are electron rich or deficient, influencing how molecules interact chemically.
Key Takeaways
- An atom sharing its lone pair in bonding effectively loses control of one electron, reducing its electron count.
- Covalent bonds split electrons between atoms, changing electron ownership and impacting formal charge.
- Atoms like nitrogen balance protons and electrons; loss of lone pair electrons leads to positive formal charge.
- Formal charge calculation highlights electron counting changes that result in positive charges after lone pair donation.
- Lone pairs carry negative charge; sharing them reduces electron density on the donor atom, creating a positive charge.
Why does nitrogen become positively charged when it shares its lone pair?
Nitrogen shares its lone pair to form a bond with H+. This causes nitrogen to lose control of one electron, reducing its electron count and resulting in a positive charge.
How does electron sharing in a covalent bond affect an atom’s charge?
In a covalent bond, electrons are split between atoms. When an atom shares its lone pair, it effectively counts fewer electrons, which can change its formal charge.
Why does nitrogen have a +1 charge in NH4+ but not in NH3?
In NH3, nitrogen has a lone pair and three bonds, counting five electrons total. In NH4+, the lone pair forms a bond, so nitrogen counts only four bonding electrons, causing a +1 charge.
What role does formal charge calculation play in explaining the positive charge?
Formal charge equals valence electrons minus bonds minus lone pair electrons. For nitrogen in NH4+, this calculation shows a +1 charge because lone pairs convert to bonding electrons.
How does sharing a lone pair reduce negative charge on the atom?
The lone pair holds negative charge. When shared in bonding, part of this negative charge is given up. This loss decreases electron density and leaves the atom slightly positive.





Leave a Comment