Why Is Hydrofluoric Acid So Dangerous if It’s a Weak Acid?
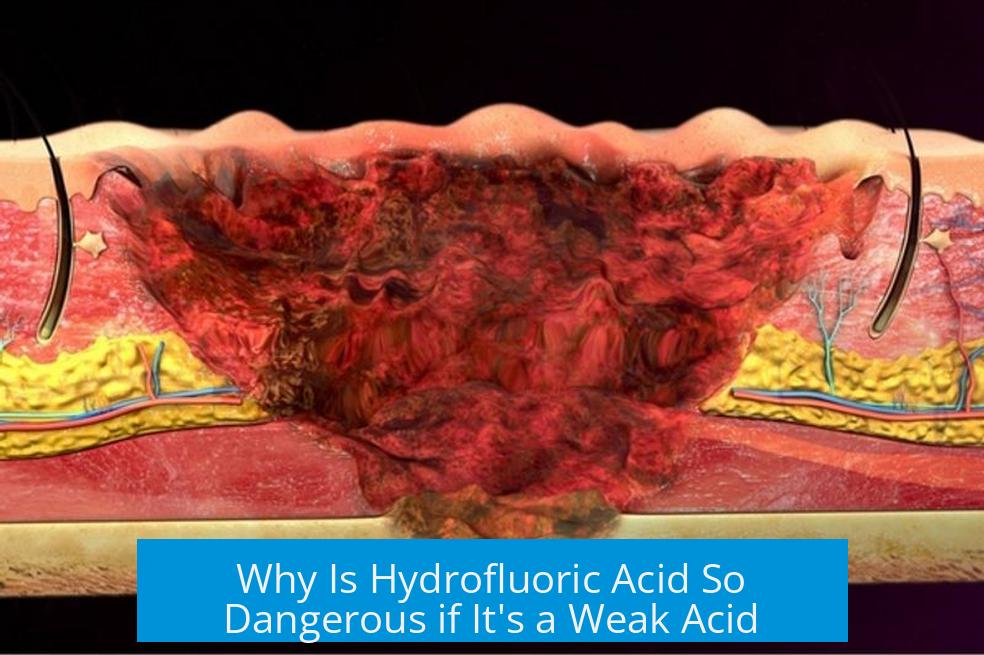
Hydrofluoric acid (HF) is extremely dangerous despite being classified as a weak acid because its danger stems not from acidity alone but from its unique chemical properties, ability to penetrate tissues rapidly, and its toxic fluoride ion that disrupts essential biological functions.
Understanding Acid Strength vs Danger

Acid strength measures how much an acid dissociates into ions in water. Strong acids, like hydrochloric acid (HCl), dissociate completely. Weak acids, including HF, dissociate partially. This does not mean weak acids are less hazardous.
Weak acids remain chemically reactive despite lower ionization. The dangers of acid exposure relate more to the nature of the acid’s ions and molecules than just their dissociation level. HF’s weak dissociation allows a significant fraction to remain as neutral, uncharged HF molecules that uniquely penetrate cells.
Hydrofluoric Acid’s Skin Penetration
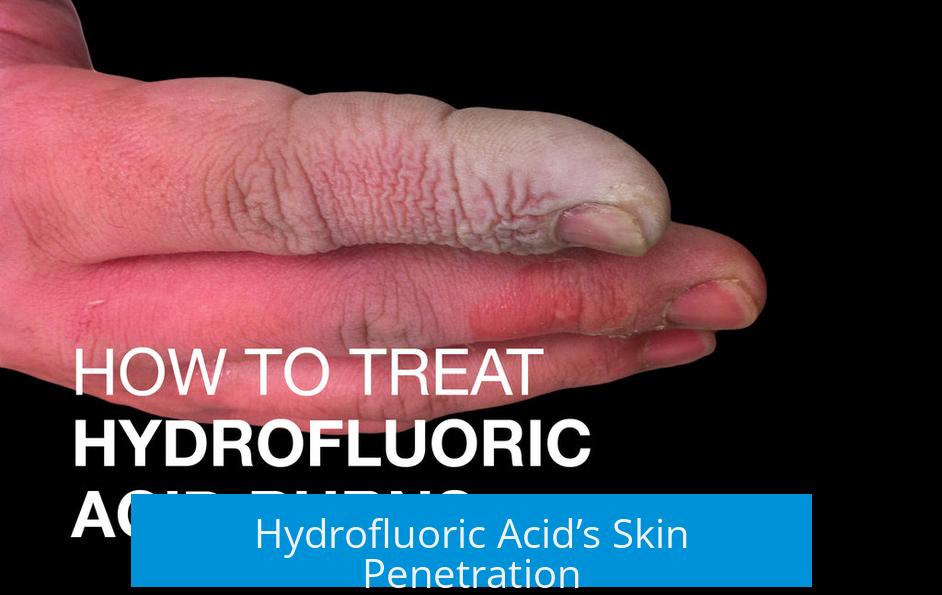
HF molecules are small and can easily pass through skin barriers. This enables the acid to reach deep tissues, muscles, and bones. Unlike stronger acids that cause immediate surface burns, HF can cause delayed symptoms allowing it to silently inflict critical internal damage.
- Penetration can take hours before pain or injury signs appear.
- Once inside, HF attacks skeletal calcium, causing decalcification.
Chemical Reactivity and Fluoride Ion Toxicity

The fluoride ion (F−) released by HF strongly reacts with calcium (Ca2+) and magnesium (Mg2+) ions in the body. This produces insoluble salts such as calcium fluoride (CaF2), which removes free calcium and magnesium from vital biological processes.
The depletion of these essential ions disrupts cell signaling and enzyme function. Fluoride additionally damages hemoglobin and mitochondrial cytochromes, impeding oxygen transport and cellular respiration.
| Effect | Outcome |
|---|---|
| Calcium precipitation | Disrupted muscle contraction and heart function |
| Magnesium precipitation | Impaired enzymatic activity |
| Hemoglobin destruction | Reduced oxygen delivery |
| Mitochondrial damage | Energy metabolism failure |
Disruption of Vital Biological Functions

Calcium ions regulate heartbeat and muscle contraction. HF exposure reduces free calcium ions, impairing these critical functions. This disruption often causes cardiac arrest and is a leading cause of death after significant HF exposure.
Moreover, HF can interfere with potassium ions critical for heart rhythm, causing dangerous arrhythmias such as bradycardia or asystole.
Prolonged and Hidden Damage
HF exposure symptoms can be delayed for hours. Initial neutralization in tissues does not end its danger, because HF and fluoride ions can re-release over time and cause ongoing damage long after contact.
In severe cases, sustained fluoride ion action necessitates medical interventions or even amputation to prevent fatal outcomes.
Corrosiveness and Handling Difficulties
Despite its weak acid classification, HF is highly corrosive. It uniquely reacts with glass and silica, etching these materials. Thus, it requires special containment in plastic or metal containers resistant to fluoride attack.
This corrosiveness extends to human tissue and minerals, contributing to its hazardous nature in industrial and laboratory settings.
Fluorine’s Electronegativity and Chemical Properties
Fluorine is the most electronegative element, forming a strong hydrogen-fluorine bond in HF. This strength partially explains why HF does not fully ionize in water, unlike other hydrogen halides.
The incomplete ionization means a substantial portion remains neutral HF molecules. These neutral molecules penetrate tissues more efficiently than ions, leading to enhanced toxicity not seen in strong acids that ionize fully.
Additionally, HF molecules form hydrogen-bonded clusters with water and fluoride ions, influencing their chemical interactions and biological penetration.
Key Takeaways
- HF’s weak acidity refers only to partial dissociation in water, not lack of hazard.
- Neutral HF molecules penetrate skin easily, reaching deep tissues and bones.
- Fluoride ions react strongly with calcium and magnesium, causing critical ion depletion.
- Calcium depletion disrupts cardiac and muscular functions, often causing fatal heart failure.
- HF damage is delayed and persistent, complicating treatment efforts.
- Hydrofluoric acid is highly corrosive to glass and tissue requiring specialized storage and handling.
- Fluorine’s electronegativity shapes HF’s unique chemical behavior and deep tissue penetration.
Why is Hydrofluoric Acid So Dangerous if It’s a Weak Acid?
Hydrofluoric acid’s danger lies not in its acidity strength but in its unique chemical behavior and biological effects. While it qualifies as a weak acid due to limited dissociation in water, HF poses grave hazards far beyond simple acid burns. This paradox often confuses many, so let’s unravel why its weak acid label doesn’t make it safe.
First off, what does “weak acid” really mean? It’s a chemistry term describing how *much* an acid breaks apart into ions in water—not a commentary on how toxic or corrosive it is. Think about it this way: a weak acid doesn’t fully release hydrogen ions, but that doesn’t mean it can’t pack a serious punch.
Acid Strength vs Danger: A Common Misunderstanding
It’s tempting to assume weak acids are harmless. That’s wrong. Acid strength measures dissociation—the split between acid molecules turning into ions in water—not their corrosive power or toxicity. Strong acids like hydrochloric acid (HCl) dissociate almost completely. For instance, if you dissolve HCl in water, nearly all of it will split into hydrogen (H+) and chloride (Cl−) ions.
Hydrofluoric acid (HF), however, remains mostly as intact HF molecules in solution, rather than separate ions. But here’s the twist: this “weakness” lets HF penetrate deeply through your skin as neutral molecules, evading immediate irritation and enabling a slow, stealthy attack on your tissues and bones. That’s unlike the sharp, quick burn of stronger acids.
Hydrofluoric Acid’s Sinister Skin Penetration
Imagine an intruder that sneaks through your defenses quietly, then wreaks havoc inside. HF acts much the same way.
Because HF doesn’t fully dissociate in water, many HF molecules remain electrically neutral. These tiny neutral HF molecules are super good at slipping through your skin’s protective barrier. While stronger acids burn the surface instantly, HF silently invades, reaching deep tissues and bones before causing visible pain or damage. It can take hours before you realize you’ve been seriously exposed.
Once inside, it attacks your nervous system, muscle tissue, and—most alarmingly—the bones. HF has the nasty nickname “bone seeker” because it actively penetrates and decalcifies bones by reacting chemically with calcium there.
The Hidden Chemical Reactivity Behind Fluoride Ion Toxicity
The danger of hydrofluoric acid extends beyond its acidity. The real villain is the fluoride ion (F−), which is highly reactive chemically. This ion isn’t just an innocent byproduct. It forms insoluble compounds with calcium and magnesium—two minerals vital for normal body function.
When fluoride binds calcium, it forms calcium fluoride (CaF2) — a compound your body can’t dissolve or use properly. This process depletes free calcium ions your body critically needs. Calcium plays a starring role in many biological processes, especially heart muscle contractions.
Fluoride also binds magnesium ions and disrupts cellular components like hemoglobin and mitochondrial cytochromes—key players in oxygen transport and cellular energy production. These disruptions can cascade into severe biochemical failures incompatible with life.
Disrupting Life’s Electrical and Structural Foundations
Did you know that your heart’s rhythm depends heavily on free calcium ions? It’s true. Calcium ions control the electrical signals that keep your heart beating steadily. If fluoride scoops up these calcium ions to form insoluble salts, your heart’s rhythm can falter.
Patients exposed to HF often suffer from bradycardia (slow heart rate), or worse, asystole, where the heart just *stops*. Even at low exposure levels, HF can steal away potassium ions, further disturbing heart function.
This high-affinity fluoride ion chemistry means that HF poisoning quickly turns from a chemical burn to a systemic, life-threatening toxin affecting organs far beyond the skin.
Why Does HF Damage Sometimes Hide in Plain Sight?
Here’s one of HF’s cruelest tricks: delayed symptoms and prolonged tissue poisoning. Because HF penetrates deeply and reacts slowly, pain and damage often don’t show up immediately after exposure. Victims might think they’re safe at first—only to experience severe pain and deep tissue damage hours later.
Inside the body, HF can be briefly neutralized but then reforms, relentlessly attacking cells until it is fully spent or causes irreversible harm. In some tragic cases, this damage necessitates amputation of the affected limb or leads to fatal heart complications.
Industrial safety teams, like those at DuPont, have explored this with training films demonstrating how even quick first aid can’t instantly halt HF’s ongoing tissue destruction.
Corrosiveness Despite “Weak” Dissociation
Hydrofluoric acid is weirdly corrosive precisely because it’s a weak acid. It cannot fully dissociate in water, yet it attacks materials aggressively. Unlike most acids that spare glass and quartz, HF actively etches glass and dissolves silica-based compounds.
This property means HF demands special handling and storage in plastic containers instead of glass ones, highlighting its hazardous nature. Chemists often say storing HF safely is a logistical puzzle because it literally eats through the usual vessels used for acids.
The Role of Fluorine’s Electronegativity
Fluorine is the most electronegative element on the periodic table. This means it strongly attracts electrons, creating a very polar H–F bond. The bond strength and polarity influence HF’s behavior in water and biological systems.
Surprisingly, this strong H–F bond partly explains HF’s weak acidity—it doesn’t fully separate into ions. Yet, this very property helps HF in a “Trojan horse” style. Most HF molecules remain neutral, so they slip past your body’s defenses easier than fully-ionized acids.
Inside your tissues, these neutral molecules dissociate slowly, releasing harmful fluoride ions right where they do the most damage. The molecular clustering and bonding in HF solutions further complicate its chemistry and toxicity.
So Why Does This Matter to You?
Simply put: don’t underestimate hydrofluoric acid because it’s “weak.” The word “weak” here is a chemistry technicality, not a safety green light.
HF exposure calls for immediate and serious medical response. Always have calcium gluconate gel (a calcium ion donor) ready if you handle HF. Applying this gel quickly after exposure helps bind fluoride ions before they wreak havoc. It’s often a life (and limb) saver.
Can you imagine an acid that can sneak under your skin, eat your bones, disrupt your heart, and strike hours after contact? HF is exactly that acid, earning a reputation as one of the most deceptively dangerous substances in any lab or industry.
Summary Table: Why is Hydrofluoric Acid So Dangerous?
| Feature | Explanation |
|---|---|
| Weak Acid Definition | Limited ion dissociation in water; most HF remains intact molecules, enabling skin penetration. |
| Skin Penetration | Neutral HF molecules easily pass through the skin, reaching deep tissues and bones. |
| Calcium Binding | Fluoride ions form insoluble calcium fluoride, disrupting calcium-dependent biological functions. |
| Biochemical Disruption | Disrupts heart rhythm, mitochondrial function, and hemoglobin, risking cardiac arrest. |
| Delayed Symptoms | Damage develops slowly, making early detection and treatment difficult. |
| Corrosiveness | Can attack glass and silica, requiring special containment such as plastic vessels. |
| Fluorine’s Electronegativity | Strong H–F bond and molecular clustering affect acidity and enable tissue penetration. |
Practical Tips For Safety
- Always wear appropriate protective gloves and gear when handling HF.
- Store HF only in approved plastic containers—not glass.
- Have calcium gluconate gel accessible and know how to apply it promptly.
- Know emergency protocols—HF exposure is a medical emergency requiring immediate care.
- Educate teams on HF’s peculiar dangers, especially its delayed symptoms.
In conclusion, hydrofluoric acid challenges the typical acid danger stereotype—it’s weak, yes, but deadly. Its ability to infiltrate your body, capture essential ions, and disrupt life-critical functions explains why it demands respect and extreme caution. Chemical knowledge here isn’t just academic; it’s a shield protecting lives.
Next time you hear “weak acid,” remember there’s more to the story. HF’s unique chemistry and biological effects make it one of the most notorious acids you wouldn’t want to meet unprepared.




Leave a Comment