Why Is This Called Octahedral When There Are Only Six Atoms Bonded to the Central Atom?
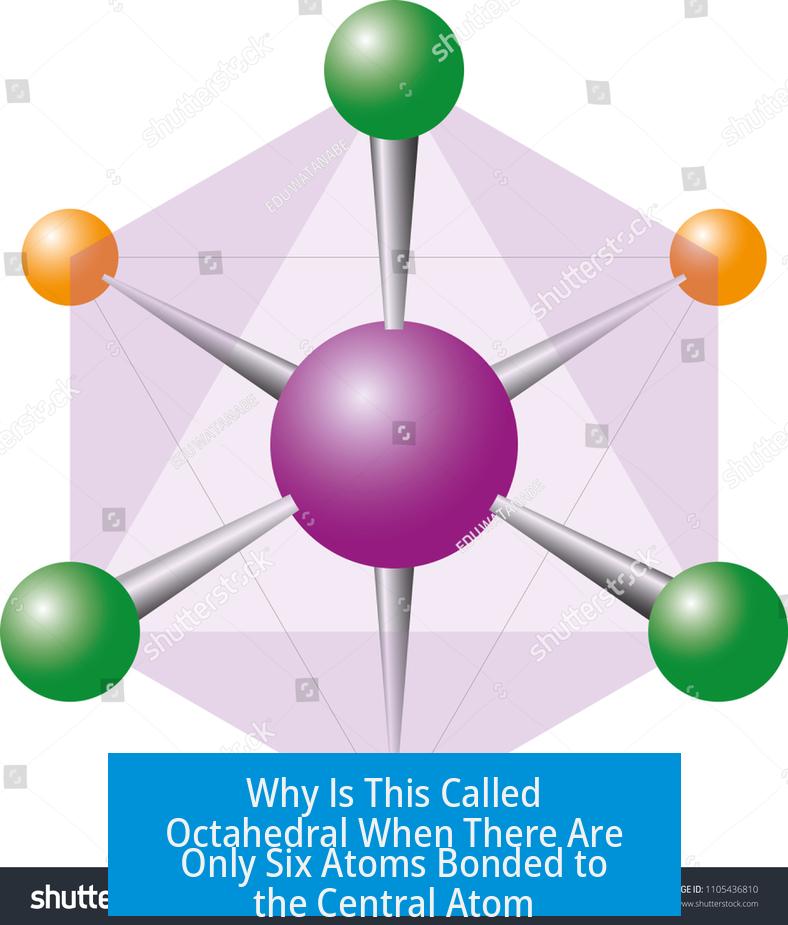
The term “octahedral” refers to the shape formed in three-dimensional space around a central atom bonded to six atoms, where these six atoms occupy positions corresponding to the six vertices of an octahedron, a polyhedron with eight faces. The naming focuses on the eight triangular faces of the shape, not the number of atoms.
1. Geometrical Basis of Octahedral Shape
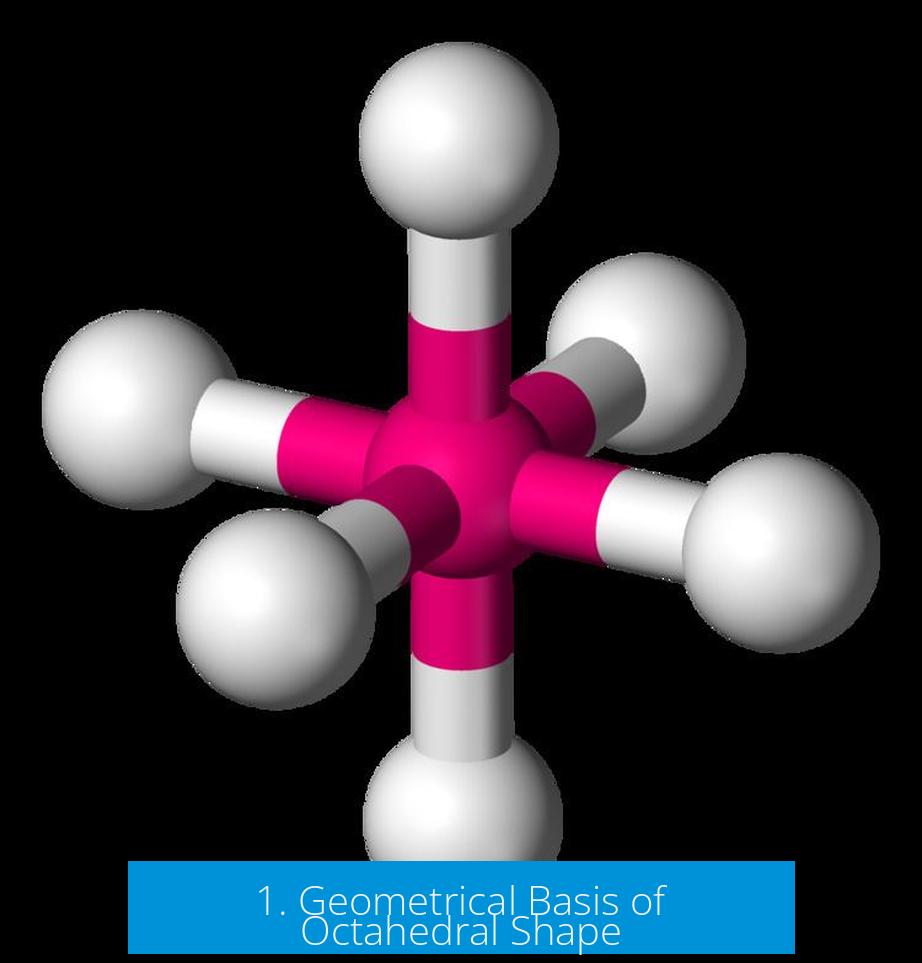
In geometry, an octahedron is a Platonic solid with 8 triangular faces and 6 vertices. When six atoms bond to a central atom symmetrically, their relative positions mimic the vertices of an octahedron. Thus, although the molecule contains six bonded atoms, their arrangement outlines a shape with eight faces.
1.1 Faces vs. Vertices: Key Distinction
- The six atoms occupy vertex positions on the 3D structure.
- The octahedron itself is defined by having 8 triangular faces.
- “Octahedral” describes the number of faces (from Greek “octa” meaning eight) and the suffix “-hedral” meaning faces or surfaces.
So, the six atoms correspond to six vertices, but the overall shape formed has eight faces. This is the fundamental reason the geometry is called octahedral despite there being only six bonded atoms.
1.2 Two Square Pyramids Joined Base-to-Base
A helpful visualization is imagining two square pyramids joined at their bases. Each pyramid has four triangular faces. Joined together, they form eight triangular faces total, the octahedron.
- Each pyramid contributes 4 triangular faces.
- Six vertices form at the corners—where atoms reside.
This model helps clarify why six atom positions correspond to eight faces.
2. Duality in Geometry and How Chemists Choose Convention
Geometry defines the relationship between different polyhedra by a concept called duality.
- The cube and octahedron are dual shapes, where the cube’s faces correspond to octahedron vertices and vice versa.
- Atoms can be imagined as located at vertex points (octahedron model) or face centers (cube model).
Chemists adopt the convention of treating atoms as vertices, leading to the octahedral description. Both dual interpretations are valid, but octahedral geometry better matches the bonding patterns seen experimentally.
3. Visual and Physical Models Confirm the Naming
Visualizing the six atoms as dots connected by bonds reveals the octahedral form:
- Connecting vertices yields eight triangular faces.
- The resulting shape resembles an eight-faced die used in tabletop role-playing games (a D8 die).
- This common physical analogy reinforces the octahedral term by focusing on faces.
This reinforces that bond positions (vertices) construct a shape defined by faces, validating the octahedral designation.
4. Molecular Models and Geometry Beyond Atoms
Models like Lewis dot structures or ball-and-stick render atom positions and bonds but often fail to show full 3D shape explicitly.
- They simplify bonding orbitals and nuclei placements.
- These models neglect the space-filling volume of the molecule.
- When fully considering geometric vectors and space, the structure fills an octahedron.
Thus, “octahedral” describes the spatial skeleton or “envelope” rather than just the literal count of bonded atoms.
5. Summary of Key Geometrical Terms
| Term | Meaning in Context |
|---|---|
| Octahedron | Polyhedron with 8 triangular faces and 6 vertices. |
| Vertices | Points where the atoms are located (6 in octahedral geometry). |
| Faces | Flat surfaces of the polyhedron (8 in octahedron). |
| Dual Polyhedron | Related shape swapping vertices and faces (cube ↔ octahedron). |
6. Why Not “Hexahedral”?
Sometimes confusion arises because the molecule has six atoms, but “hexahedral” is not used because “hedral” describes faces, not vertices.
Hexahedron refers to shapes with six faces, like a cube, which is a different geometry with eight vertices—not the case here.
Therefore, bonding to six atoms arranged at octahedral vertices forms the shape named for its eight faces, consistent with the octahedron.
Key Takeaways
- “Octahedral” references the eight faces of the shape, not the number of bonded atoms.
- Six atoms arrange at the six vertices of an octahedron, giving the molecule its symmetry.
- Visualizing connections between atoms forms two square pyramids base-to-base, totaling eight faces.
- In geometry, octahedron and cube are dual shapes; chemists adopt the octahedron view with atoms as vertices.
- Molecular models simplify bonds but octahedral refers to full spatial symmetry and shape.
- This naming is standard in coordination chemistry for molecules like SF6 or coordination complexes with six ligands.
Why is the shape called octahedral if there are only six atoms bonded to the central atom?
The term “octahedral” comes from the number of faces of the shape, not the number of atoms. Six atoms form the vertices of an octahedron, which has 8 triangular faces.
How do six atoms form a shape with eight faces?
Connecting the six atoms forms two square pyramids joined base-to-base. Together, these create a shape with eight triangular faces, called an octahedron.
What does the “-hedral” suffix mean in octahedral?
“-Hedral” refers to the faces or surfaces of a solid. Octahedral means having eight faces, regardless of the number of vertices or atoms bonded.
Why do chemists say octahedral, not cubic, for six bonded atoms?
Due to geometric duality, six atoms can be viewed as vertices of an octahedron or faces of a cube. Chemists choose the octahedral convention, treating atoms as vertices with eight faces surrounding them.
Can you explain the shape using common objects?
The octahedral shape resembles an eight-sided dice used in role-playing games. It has 8 flat surfaces formed by connecting six corner points, just like the bonded atoms.



Leave a Comment