Aldol and Claisen Condensations: A Detailed Comparison and Mechanisms
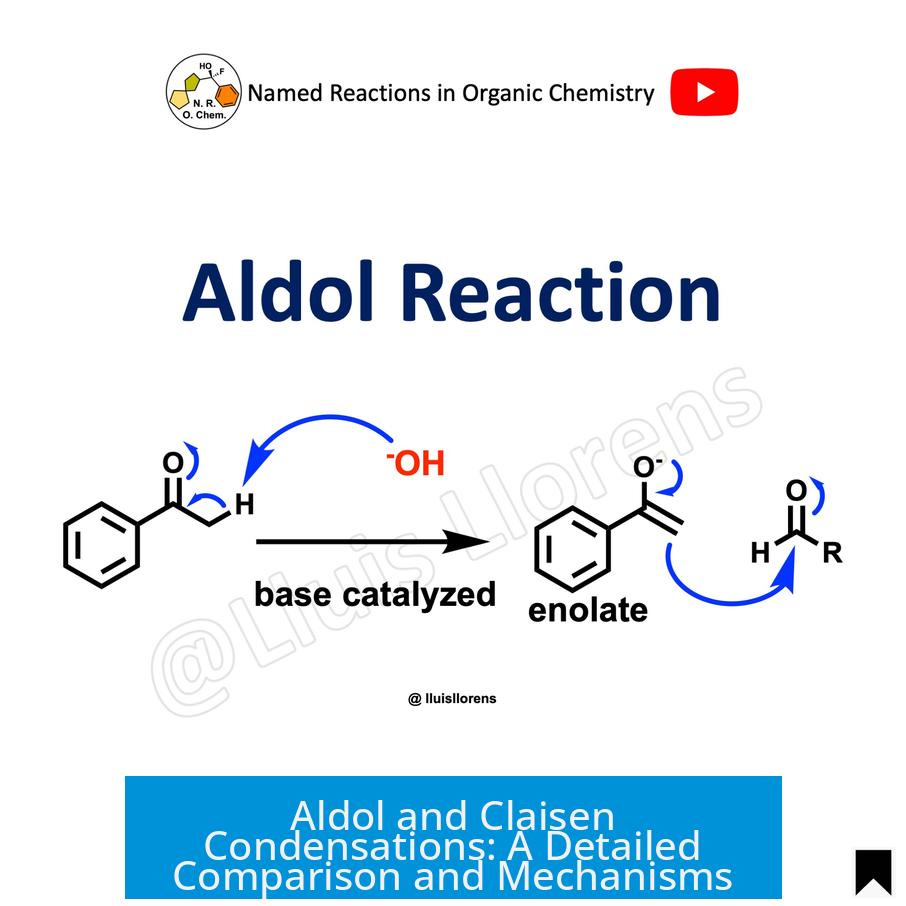
The Aldol and Claisen condensations are two essential reactions in organic chemistry that form carbon-carbon bonds by combining carbonyl compounds under basic or acidic conditions, each with unique mechanisms and conditions.
Overview of Aldol Reaction and Aldol Condensation
The aldol reaction involves connecting two carbonyl compounds, typically aldehydes or ketones, to form a β-hydroxy carbonyl compound called an “aldol.” This product contains a hydroxy group (-OH) located two carbons away from the aldehyde or ketone group. The reaction is a cornerstone in building complex molecules with new carbon-carbon bonds.
When the β-hydroxy carbonyl compound formed undergoes heating with a base, it typically dehydrates via an E1CB elimination mechanism, producing an α,β-unsaturated carbonyl compound. This elimination product defines the aldol condensation. The dehydration step happens readily and often proceeds under the same reaction conditions, which is why the terms “aldol reaction” and “aldol condensation” sometimes overlap.
Mechanism of Aldol Reaction
- Under basic catalysis, an enolate ion (formed via deprotonation at the alpha hydrogen) attacks the carbonyl carbon of another molecule, forming a new carbon-carbon bond.
- Under acidic conditions, the reaction proceeds through protonated intermediates where an enol tautomer attacks the electrophilic carbonyl group.
Special Cases in Aldol Reactions
- Some reactions, such as the aldol reaction of acetone, do not favor product formation, with equilibrium favoring reactants (only 2% aldol product under normal conditions).
- Cross-aldol reactions involve two different carbonyl compounds but can produce mixtures unless one component cannot form enolates (e.g., benzaldehyde).
- Stereoselectivity depends mainly on the enolizing metal counterion and the enolate isomer, often explained by the Zimmerman–Traxler transition state model.
Claisen Condensation: Definition and Requirements
The Claisen condensation is a reaction that couples two esters or an ester and a ketone under strong basic conditions to create a β-ketoester or β-diketone. Like the aldol reaction, it forms a new carbon-carbon bond, but it distinctly requires a strong base and proceeds with the formation and subsequent deprotonation of an intermediate to drive the reaction forward.
Final Deprotonation Step
- A critical difference from aldol condensation is the necessary final deprotonation of the β-ketoester product using sodium ethoxide or a stronger base to stabilize the product and shift equilibrium.
- Without this step, the reaction often tends to favor the reactants.
Handling Esters with Single Alpha Hydrogens
- If only one alpha proton is present on the ester, the reaction equilibrium usually favors starting materials because deprotonation and subsequent steps are less favorable.
- Using very strong bases like triphenylmethide, the reaction can proceed, but it requires two equivalents of the base because the strong base also deprotonates the β-ketoester product’s alpha hydrogen.
Comparison Between Aldol and Claisen Condensations
| Aspect | Aldol Condensation | Claisen Condensation |
|---|---|---|
| Substrates | Aldehydes or ketones | Esters or esters with ketones |
| Product | β-hydroxy aldehyde or ketone (aldol), later dehydration to α,β-unsaturated carbonyl | β-ketoester or β-diketone |
| Base Required | Moderate bases like hydroxide or alkoxide; deprotonation of product not always necessary | Strong bases such as sodium ethoxide or stronger; final product must be deprotonated |
| Alpha Hydrogen Requirement | At least one alpha hydrogen; multiple alpha hydrogens preferred | At least two alpha hydrogens preferred; strong bases needed if only one |
| Equilibrium Favorability | May favor starting materials (example: acetone aldol) | Usually requires final deprotonation to push equilibrium toward products |
| Reaction Mechanism | Nucleophilic enolate attack on carbonyl C; acid or base catalyzed | Enolate formation followed by nucleophilic acyl substitution and deprotonation |
Advanced Topics in Aldol Reactions
- Cross-Aldol Reactions: These involve two different carbonyl compounds and can be controlled kinetically using strong bases like LDA at low temperatures.
- Stereoselectivity: The metal counterion and enolate configuration determine the stereochemical outcome. Zimmerman–Traxler transition state models explain product stereochemistry via six-membered ring intermediates.
- Reaction Control: Reaction conditions including temperature, base strength, and choice of substrates drastically affect yields and selectivity.
Key Takeaways
- Aldol condensation forms β-hydroxy carbonyl compounds and proceeds via enolate nucleophilic attack; dehydration usually follows to yield α,β-unsaturated products.
- Claisen condensation requires strong bases and a critical final deprotonation of the β-ketoester product to drive the reaction forward.
- The presence and number of alpha hydrogens affect both reactions’ equilibria and products significantly.
- Cross-aldol reactions and stereochemical control depend on substrates and reaction conditions, guided by mechanisms like Zimmerman–Traxler.
- Understanding these mechanisms facilitates carbon–carbon bond formation, a fundamental process in organic synthesis.
Aldol and Claisen Condensation: The Dynamic Duo of Carbon-Carbon Bond Formation
What exactly are Aldol and Claisen condensations? In the realm of organic chemistry, these reactions stand as fundamental tools for building carbon-carbon bonds, crucial for synthesizing complex molecules. To put it simply: Aldol condensation combines two carbonyl compounds to form a new molecule featuring a β-hydroxy carbonyl group, which can then dehydrate to an alpha, beta-unsaturated carbonyl. Claisen condensation, on the other hand, involves esters reacting under basic conditions to yield β-ketoesters or β-diketones. But there’s more—many movers and shakers under the hood affect how these reactions proceed, making them endlessly fascinating.
Aldol Reaction: The Friendly Matchmaker of Carbonyls
Imagine two carbonyl compounds, aldehydes or ketones, meeting in a lab flask. When a base swoops in, it deprotonates the α-carbon of one molecule to form an enolate ion—a kind of supercharged carbon nucleophile. This nucleophile then attacks the carbonyl carbon of the other molecule. Voilà! You get a β-hydroxy carbonyl compound, the “aldol.”
What makes this so special? The aldol reaction is among the most common ways chemists form new carbon-carbon bonds. If you think of organic chemistry as molecular construction, aldol reaction is one of the primary ways to lay bricks.
The Aldol Condensation Twist
Heat things up in the presence of a base and the aldol tends to say goodbye to its hydroxyl group and a hydrogen—literally shedding water in a dehydration step. This elimination reaction, via an E1CB mechanism, forms a conjugated α,β-unsaturated carbonyl compound—chemists’ favorite building block.
But here’s an intriguing rub: sometimes, the equilibrium just tips towards the starting materials. A classic example is acetone undergoing aldol condensation, where only about 2% of the aldol product forms under normal conditions. Despite abundant α-hydrogens, the system prefers playing it safe.
Curious why? The relatively low electrophilicity of acetone and the reversibility of the aldol addition keep the reaction from running full steam ahead.
When Cross-Aldol Gets Complicated
Mix two different carbonyls, and you may get a cocktail of products—complicated mixtures that no one really wants on their hands. However, clever chemists avoid messy mixtures by choosing one partner that cannot form enolates. Formaldehyde and benzaldehyde are common examples; they only act as electrophiles but never nucleophiles.
Want to really control the reaction? Use kinetic control. Generate the enolate quantitatively with a strong base like LDA at low temperatures (-78 °C), then add the electrophile. This way, you get a clean product with minimal fuss.
Stereoselectivity: The Shape Shifter
Not all aldol products are created equal. Stereochemistry—the 3D shape of molecules—matters a lot. What controls this? The metal counterion on the enolate is a chief player. Shorter metal-oxygen bonds tighten the reaction’s transition state and crank up stereoselectivity.
Another key factor: the enolate’s geometry. If it’s an E isomer, you usually get anti-aldol products; if Z, syn products. Guess what predicts these outcomes often? The Zimmermann–Traxler model, which imagines the transition state as a six-membered ring to foresee how things will line up.
The Claisen Condensation: For the Esters Among Us
When esters enter the fray, Claisen condensation takes the spotlight. Let’s break it down: using sodium ethoxide as a base, esters with α-hydrogens react to form β-ketoesters—vital intermediates for numerous synthetic pathways.
The Vital Deprotonation Step
Don’t skip this part! The final deprotonation using sodium ethoxide is mandatory for Claisen condensation to proceed. Think of it as completing the handshake in a business deal—both parties must fully commit.
Watch Out for Alpha Hydrogens
But what if your ester only has one α-hydrogen? Typically, the reaction then pushes back and favors the reactants. The system isn’t confident enough to move forward. Here’s where chemistry gets interesting.
Going Stronger Than Ethoxide
If you want to force the reaction with esters having just a single α-hydrogen, stronger bases come to the rescue. Enter triphenylmethide, a base so powerful it can deprotonate the harder-to-reach α-hydrogen and even the ketoester’s α-hydrogen after condensation.
- Important: Using such a strong base requires two equivalents.
- Why? Because this intense base removes protons twice—once to make the enolate and once afterward to stabilize the ketoester product’s α-hydrogen.
This makes the reaction more challenging but still feasible with the right conditions.
Putting It All Together: Practical Tips and Insights
Both aldol and Claisen condensations are powerful in organic synthesis but demand careful control and understanding of the reaction conditions. Here are some tips:
- Base Choice Matters: Sodium ethoxide suits many Claisen condensations, but for stubborn esters, stronger bases like triphenylmethide are essential.
- Monitor Alpha Hydrogens: More α-hydrogens make the reaction smoother. Only one α-hydrogen? Prepare to adjust your strategy.
- Temperature and Equilibrium: Aldol products sometimes revert; heating and continued removal of water push equilibrium towards condensation.
- Stereochemical Control: Utilize metal counterions and enolate isomers to tailor product stereochemistry.
- Crossing Aldols: Use non-enolizable electrophiles or kinetic control to get clean cross-aldol products.
Real-world Example: Formation of β-Ketoesters via Claisen Condensation
Suppose you’re synthesizing a β-ketoester for pharmaceutical research. Starting with ethyl acetate, you perform Claisen condensation using sodium ethoxide. There is no drama here because ethyl acetate has two α-hydrogens, making enolate formation straightforward.
But switch ethyl acetate to an ester harboring only one α-hydrogen, and your yields plummet unless you bring a stronger base (like triphenylmethide) into the mix, using two equivalents to ensure the reaction goes forward. This attention to detail makes all the difference.
Why Should You Care?
The practical takeaways? These condensations enable chemists to connect molecular fragments cleanly and efficiently, forming key C-C bonds. They are the backbone of creating new molecules from scratch or modifying existing ones. Without understanding the nitty-gritty of base strength, α-hydrogen availability, equilibrium tendencies, and stereoselectivity, you might as well toss a coin and hope for your favorite product.
So, are you ready to harness the power of Aldol and Claisen condensations in your next synthesis?
Keep exploring these mechanisms, consider the delicate balance of conditions, and don’t be afraid to adjust your bases and temperatures. The more you know these reactions’ quirks, the more chemist-like power you wield.
What is the role of the final deprotonation in Claisen condensation?
In Claisen condensation with sodium ethoxide, the final deprotonation is essential. It stabilizes the ketoester product by removing an acidic proton. Without this step, the reaction does not proceed efficiently.
Why does Claisen condensation fail with esters having only one alpha hydrogen?
When an ester has only one alpha hydrogen, the reaction favors starting materials. This is because the equilibrium lies towards the reactants, making condensation difficult under normal conditions.
How can Claisen condensation be performed on esters with a single alpha hydrogen?
You can use a much stronger base than sodium ethoxide, such as triphenylmethide. Two equivalents of the strong base are needed to fully deprotonate and drive the reaction forward.
Does aldol condensation always require a deprotonation step?
No, aldol condensation does not necessarily require a deprotonation step. In many cases, it proceeds without it, unlike Claisen condensation.
Why does aldol condensation of acetone give low yields of aldol product?
The equilibrium for acetone aldol condensation favors the starting materials. Even with multiple alpha hydrogens, under ordinary conditions only about 2% of the aldol product forms at equilibrium.
How does the stereoselectivity of aldol reactions get influenced?
The enolizing metal counterion mainly controls stereoselectivity. Also, the enolate isomer affects whether anti or syn products form. The Zimmerman–Traxler model helps predict these stereochemical outcomes.




Leave a Comment