Does Benzene Have Two Resonance Structures?
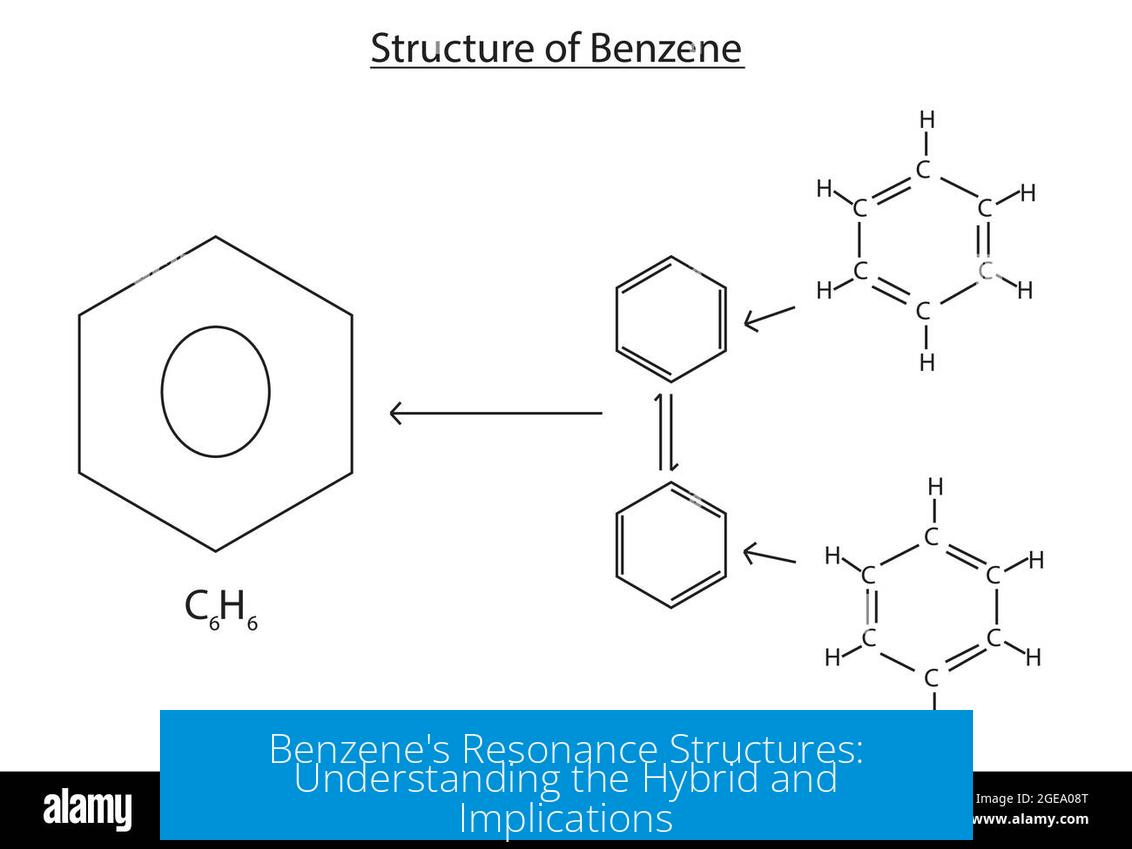
Benzene has two main resonance structures, which are degenerate and contribute equally to its true resonance hybrid structure. These two Lewis structures show alternating single and double bonds arranged in different positions around the six-carbon ring. Neither structure exists alone; instead, benzene’s true form is a hybrid, reflecting an equal mix of both.
Nature of Benzene’s Resonance Structures
- Benzene’s two principal resonance structures differ only in the location of the double bonds.
- They are called degenerate because both have equal stability and energy.
- Though more resonance forms exist, only these two significantly contribute to the hybrid.
These resonance forms are best viewed as extremes rather than real molecular entities. Each shows alternating double and single bonds, but the actual molecule does not have fixed double bonds in these positions.
Resonance Hybrid and True Molecular Structure
The actual benzene molecule is a resonance hybrid of the two structures. This hybrid features six equal bonds between carbon atoms, each with a bond order of approximately 1.5.
| Property | Resonance Structures | Resonance Hybrid (True Benzene) |
|---|---|---|
| Bond length | Alternates between single and double | Equal length, intermediate between single and double |
| Bond order | 1 or 2 | ~1.5 (some calculations suggest up to 1.66) |
| Stability | Less stable than hybrid | Most stable form due to delocalization |
Molecular orbital theory refines this picture, predicting bonds slightly stronger than 1.5 but weaker than a full double bond.
Chemical Implications of Resonance
The resonance hybrid explains benzene’s unusual chemical behavior. For example, identical substituted toluenes with substitutions at positions 2 and 6 confirm that these positions are equivalent due to bond delocalization.
- Resonance causes the conjugated π-electron system to extend throughout the ring.
- This extended conjugation underlies benzene’s aromaticity, enhancing stability.
- Positions in the ring are equivalent because bonds are intermediate in character, not fixed.
Clarifications About Resonance Structures
It is incorrect to consider resonance structures as isomers. They do not represent isomeric forms but rather hypothetical extremes used to describe the resonance hybrid. Treating them as distinct molecules leads to misunderstanding benzene’s true nature.
Key Takeaways
- Benzene has two main resonance structures that are degenerate and equally stable.
- The true molecular structure is a resonance hybrid featuring equal bond lengths and bond order around 1.5.
- Resonance explains benzene’s aromaticity and its chemical properties.
- Resonance structures are not isomers but conceptual tools to depict electron delocalization.
How many resonance structures does benzene mainly have?
Benzene mainly has two resonance structures. These two are equal contributors to the true structure. They look the same but have double bonds in alternating positions.
Are the two resonance structures of benzene real compounds?
No, resonance structures are not real compounds. They are hypothetical extremes. The actual structure is a hybrid, blending both forms equally.
What is the bond order between carbon atoms in benzene?
The bond order in benzene is about 1.5 between each carbon pair. This means the bonds are intermediate between single and double bonds, reflecting resonance.
Why do benzene’s resonance structures not represent isomers?
Resonance structures are not isomers. They depict different electron arrangements of the same molecule, not different molecules. Treating them as isomers is misleading.
How does resonance explain the equivalence of positions in substituted benzene?
Resonance causes bond electrons to be delocalized. Positions like 2 and 6 in methylbenzene show identical properties because the molecule is a hybrid of resonance forms.




Leave a Comment