What Does DMSO Do in an SN2 Reaction?
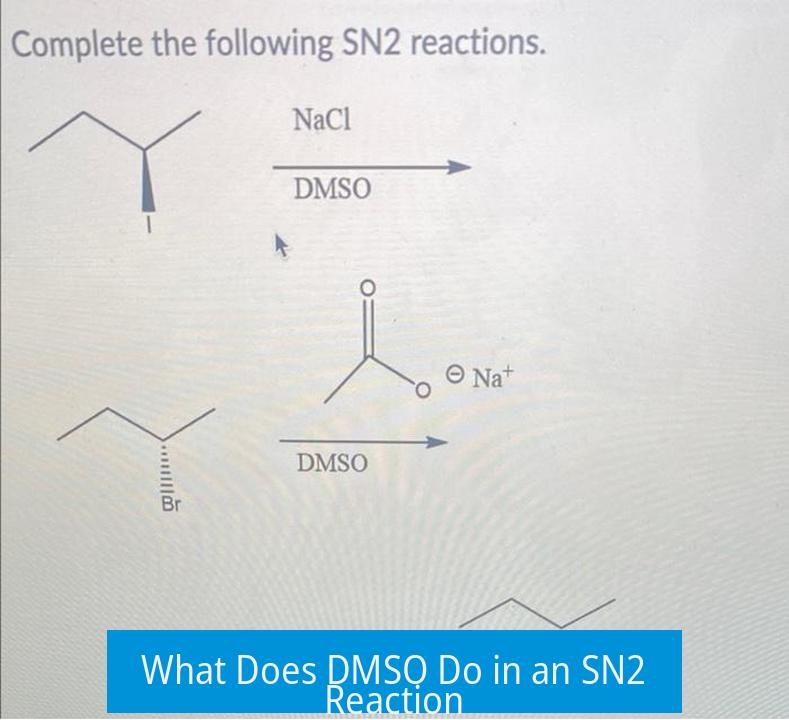
DMSO serves primarily as a polar aprotic solvent that enhances the nucleophilicity of the nucleophile by solvating the counterion but not the nucleophile itself, thereby accelerating the SN2 reaction without changing its fundamental mechanism.
Understanding DMSO’s Role as a Solvent
DMSO (dimethyl sulfoxide) is a polar aprotic solvent widely used to facilitate SN2 reactions. Its molecular structure features a sulfoxide group (S=O) where the oxygen atom carries a partial negative charge and the sulfur atom carries a partial positive charge. This polarity allows DMSO to interact effectively with ions.
Polar Aprotic Nature Supports SN2 Pathway
DMSO does not form hydrogen bonds with nucleophiles because it lacks acidic protons. Unlike polar protic solvents (which hydrogen-bond to nucleophiles and reduce their nucleophilicity), DMSO’s inability to coordinate strongly with nucleophiles leaves them relatively “free” and highly reactive.
- Polar aprotic solvents enhance nucleophilicity by not strongly solvating anions.
- DMSO’s polar nature supports solvation of cations, not anions.
- This trait favors the SN2 mechanism over competing mechanisms like E2 or SN1.
Specific Solvation: Cations vs. Anions
The partial negative oxygen in DMSO stabilizes positively charged ions, such as sodium (Na+), through electrostatic interactions. This stabilization reduces ion pairing between a nucleophile (often an anion like ethoxide, CH3CH2O−) and its metal cation.
By solvating the cation, DMSO “frees” the anion nucleophile to exert its full negative charge when attacking the electrophilic carbon.
| Ion | Interaction with DMSO | Effect on Reaction |
|---|---|---|
| Na+ | Strongly solvated by oxygen in DMSO | Reduced ion pairing, enhanced nucleophile activity |
| CH3CH2O− | Less solvated due to steric hindrance around sulfur | Retains strong nucleophilicity for SN2 attack |
Due to steric hindrance from the methyl groups on sulfur, DMSO solvates cations more efficiently than bulky anions, indirectly boosting nucleophile strength.
DMSO’s Effect on the Reaction Mechanism and Rate
DMSO does not alter the fundamental SN2 mechanism; it facilitates the reaction by increasing the nucleophile’s activity and solubility. The reaction proceeds via a backside attack with simultaneous displacement.
The solvent boosts reaction rate but does not switch or fundamentally modify the chemical process.
DMSO and Competing Reaction Pathways
While DMSO favors SN2 by enhancing nucleophilicity, reaction conditions and substrate type influence whether SN2 or elimination (E2) predominates.
- Secondary substrates with strong bases like ethoxide often undergo E2, especially at higher temperatures.
- DMSO’s presence does not prevent elimination; heat or substrate structure can still favor E2.
- Even at room temperature, SN2 on certain secondary carbons with alkoxides can yield less than 15% substitution.
Therefore, solvent choice alone does not guarantee exclusive SN2 outcome.
How to Identify DMSO in Reaction Schemes
DMSO usually appears below the reaction arrow as the solvent. Recognizing it helps to infer the propensity for SN2 by understanding its solvent properties.
- Common polar aprotic solvents appearing similarly include DMF, THF, DCM, and CCl4.
- Solvent choice is a strategic part of reaction design aiding certain pathways.
Clarifications and Misconceptions
Some confusion exists around whether DMSO oxidizes nucleophiles (e.g., “sulfoxidizes” them). In SN2 contexts, DMSO acts as an inert solvent and does not chemically oxidize reactants.
Focus should be on conditions and reagents above the arrow in a reaction scheme to explain regio- or stereochemical differences rather than attributing such changes to the solvent.
Summary
- DMSO is a polar aprotic solvent that enhances SN2 reaction rates by solvating cations but not nucleophilic anions.
- It improves nucleophile reactivity without altering the SN2 mechanism.
- DMSO’s steric and electronic environment favors ionic separation, freeing nucleophiles for attack.
- Reaction temperature and substrate structure often have a larger influence on whether elimination or substitution dominates.
- DMSO does not oxidize nucleophiles in typical SN2 reactions; it functions purely as a solvent.
What role does DMSO play as a solvent in an SN2 reaction?
DMSO is a polar aprotic solvent. It does not solvate nucleophiles through hydrogen bonding, which increases nucleophile strength. This helps nucleophiles attack the electrophilic carbon more effectively in SN2 reactions.
How does DMSO improve nucleophile reactivity in SN2 reactions?
DMSO solvates positively charged cations like Na+ using its oxygen’s partial negative charge. This frees the nucleophile, allowing its full negative charge to attack the substrate more efficiently in an SN2 pathway.
Does DMSO change the SN2 mechanism itself?
No. DMSO acts to speed up the reaction but does not alter the fundamental SN2 mechanism. It supports the nucleophile and reactants but doesn’t cause a different reaction pathway.
Can DMSO prevent competing elimination reactions (E2) in SN2 setups?
DMSO can promote SN2 but cannot always prevent elimination. Heat or the substrate structure may still favor E2, regardless of DMSO being the solvent.
Why is DMSO preferred over polar protic solvents for SN2?
Polar protic solvents stabilize nucleophiles by hydrogen bonding, lowering their reactivity. DMSO, being polar aprotic, avoids this and better supports stronger nucleophiles needed for SN2.




Leave a Comment