Nucleophile Strength Pattern for SN1 and SN2 Reactions
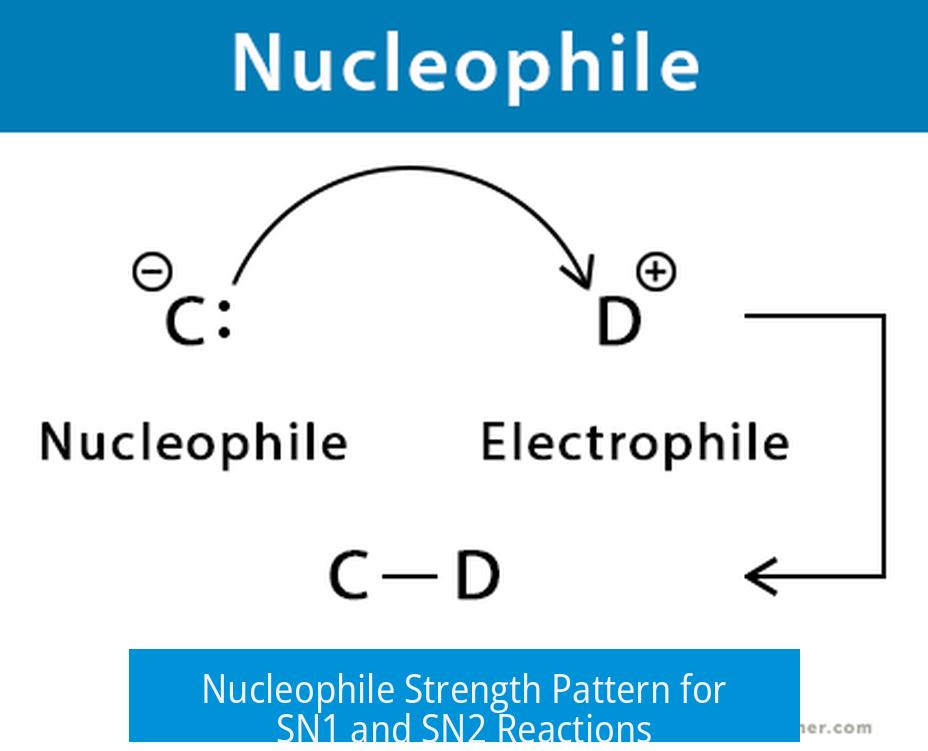
The nucleophile strength pattern differs significantly between SN1 and SN2 mechanisms due to their distinct reaction pathways and rate-determining steps. SN2 reactions demand strong nucleophiles for the direct backside attack on the electrophile, while SN1 reactions depend mostly on carbocation stability, making nucleophile strength less critical.
Mechanistic Differences and Their Impact

SN2 reactions proceed through a single step where the nucleophile attacks the electrophilic carbon directly. Nucleophile strength governs the reaction rate because it lowers the activation energy. Conversely, SN1 reactions occur in two steps, with carbocation formation being rate-limiting. The transition state’s energy depends heavily on carbocation stability rather than the nucleophile.
Substrate Structure Controls Pathway Preference

- Primary (1°) substrates: Steric hindrance is minimal, favoring SN2 pathways. Here, strong nucleophiles increase the reaction rate.
- Tertiary (3°) substrates: Steric hindrance blocks SN2, favoring SN1. Carbocation forms readily, so nucleophile strength is less important.
- Secondary (2°) substrates: Both SN1 and SN2 are possible. Nucleophile strength and solvent choice guide the mechanism.
Nucleophile Characteristics Affecting Strength
A nucleophile’s strength depends on the electron-donating atom’s charge and size. Negatively charged nucleophiles are stronger. Larger atoms with more diffuse electron clouds (e.g., I− vs F−) tend to be better nucleophiles due to weaker solvation.

Solvent Influence on Nucleophile Strength and Mechanism
Protic solvents stabilize carbocations through hydrogen bonding, thus favoring SN1 by lowering carbocation energy. Such solvents also hinder nucleophiles by solvation, reducing their effectiveness in SN2. Aprotic solvents do not stabilize carbocations but allow strong nucleophiles to stay reactive, favoring SN2.

Relation to Basicity
Nucleophilicity often correlates somewhat with basicity but differs kinetically. Nucleophiles participate in faster, kinetically controlled reactions, while bases govern thermodynamic outcomes. This distinction is more prominent in SN2 reactions.

Summary of Key Points
- SN2: Requires strong nucleophiles – negatively charged and larger atoms increase strength; steric hindrance matters.
- SN1: Nucleophile strength has minor effect; carbocation stability and protic solvents dominate.
- Substrate type: Primary favors SN2; tertiary favors SN1; secondary depends on nucleophile and solvent.
- Solvent: Protic solvents promote SN1; aprotic solvents favor SN2 by preserving nucleophile strength.
Can Someone Please Explain Nucleophile Strength Pattern for SN1 and SN2 Reactions?

Alright, here’s the burning question: What’s the nucleophile strength pattern for SN1 and SN2 reactions? Let’s cut to the chase and get that answer on the table first:
For SN2 reactions, nucleophile strength is a major player—it directly affects how fast and effectively the nucleophile attacks the electrophile. In contrast, SN1 reactions hardly care about nucleophile strength; instead, they focus on carbocation stability, with nucleophiles showing up fashionably late after the rate-determining step.

Why does one reaction obsess over nucleophile strength while the other doesn’t? Let’s dive into the fascinating details that reveal this chemical drama.
Breaking Down the Mechanism Drama: SN1 vs. SN2

Imagine two parties where nucleophiles behave very differently. SN2 is an action-packed single-step concert. The nucleophile crashes directly onto the electrophile, pushing off the leaving group like a fierce headbanger. Here, the nucleophile’s punch matters a lot. The stronger it is (think of a strapping bouncer), the easier it forces the leaving group out.
On the flip side, SN1 is a slow burn drama unfolding in two acts. First, the leaving group leaves solo, creating a carbocation star who steals the spotlight. Only then does the nucleophile swoop in for the encore. Since the star carbocation’s stability determines how fast the show progresses, the nucleophile’s strength barely influences the pace.
| Aspect | SN2 | SN1 |
|---|---|---|
| Rate-Determining Step | One-step: Direct attack | Two-step: Carbocation formation |
| Nucleophile Role in Rate | Major; strength lowers activation energy | Minor; carbocation stability dominates |
| Electrophile Preference | Primary favored (little steric hindrance) | Tertiary favored (carbocation stability) |
The Electrophile’s Substitution Level: Where It All Begins
You can’t expect nucleophiles to do a solo without the right stage. The electrophile’s substitution pattern sets the scene. Primary carbons, with minimal crowding, welcome nucleophiles in SN2 style. Here, a strong nucleophile’s strength shines.
But tertiary carbons? Oh, they are like VIP guests with a thick entourage—the leaving group leaves first, forming a carbocation star for an SN1 reaction. The nucleophile’s strength? It’s like background music; present, but not setting the mood.
Secondary carbons? They’re the wildcard, neither fully open nor overly crowded. You need to consider the nucleophile and solvent to predict the pathway accurately for them.
What Makes a Nucleophile “Strong”? The Electron Donor’s Secret Weapons
Wondering how to size up a nucleophile? It’s simpler than your last social media profile review. Look at the atom donating the electrons:
- Negative charge: More negative means more eager to donate electrons.
- Atomic radius: Bigger atoms generally deliver a stronger, faster hit due to polarizability.
For example, iodide (I−) is a champion nucleophile in polar aprotic solvents, bigger and more polarizable than fluoride (F−). So, in SN2 reactions, iodide kicks open the door faster.
Solvent Effects: The Unsung Matchmaker Influencing Reaction Pathway
Solvents often step behind the scenes, but they have a starring role in deciding SN1 vs. SN2. Protic solvents, which hydrogen-bond like they’re networking at a conference, stabilize carbocations and slow down nucleophiles by surrounding them. This environment cheers for SN1, where carbocation stability is king.
In contrast, aprotic solvents keep the nucleophile spry and ready. Picture a nucleophile doing a sprint with no interference. These solvents favor SN2 by allowing strong nucleophiles to attack without being bogged down.
Nucleophilicity vs. Basicity: Speed Demon and Stability Buff
Heads up! Nucleophilicity and basicity often get confused. They’re related but play different roles. Nucleophilicity is all about kinetics—how fast a nucleophile strikes. Basicity is thermodynamics-focused—how stable the end product is after proton abstraction.
For SN2, the kinetic factor dominates. A nucleophile that acts fast (high nucleophilicity) will win the race. For SN1, neither nucleophilicity nor basicity really run the show’s timing.
Summing It All Up: Your Nucleophile Strength Cheat Sheet
- SN2 Reactions: Strong nucleophiles (negatively charged, larger radius) are decisive players. They lower the activation energy and enable direct attack. Primary substrates are your playground here.
- SN1 Reactions: Focus on carbocation stability and the nature of the substrate. Nucleophile strength takes a backseat. Protic solvents make carbocations comfy and stabilize the process.
- Your Strategy Should Be: First, check your substrate – primary hints SN2, tertiary hints SN1. Secondary is case-by-case and needs more detective work.
- Next, consider nucleophile strength. Only crucial if SN2 is possible. Strong? Favor SN2. Weak? Maybe SN1 sneaks in.
- Lastly, consider solvents. Aprotic solvents boost nucleophile punch; protic solvents cozy up carbocations.
Practical Example: How Does This Play Out?
Take bromocyclohexane as your substrate. It’s secondary, so the pathway isn’t obvious. If you toss in a strong nucleophile like hydroxide (OH−) in an aprotic solvent, you’ll likely see SN2, because the nucleophile attacks directly.
Switch to a weak nucleophile like water (H2O) in a protic solvent, and carbocation formation becomes more favorable. The reaction drifts to SN1, where nucleophile strength matters less, and the carbocation intermediate calls the shots.
Final Thoughts: Don’t Let Nucleophile Strength Intimidate You
Nucleophile strength isn’t a universal boss over substitution reactions. It holds mighty power in SN2, but it’s more like a casual party guest at an SN1 gathering. Focus on the substrate’s substitution level, the solvent’s temperament, and then the nucleophile’s muscle. This trio will help you predict exactly how the dance will go down.
In summary, remember this mantra:
Check your electrophile first, size up the nucleophile second, and adjust for the solvent last.
With this approach, nucleophile strength becomes less mystifying and more like your trusty sidekick in organic chemistry adventures.
What role does nucleophile strength play in SN2 reactions?
In SN2 reactions, nucleophile strength is crucial. Strong nucleophiles attack the electrophile directly, lowering activation energy. Strong means negatively charged and larger atomic radius.
Does nucleophile strength affect SN1 reactions the same way as SN2?
No. Nucleophile strength is less important in SN1. The reaction depends mostly on carbocation stability. The nucleophile usually attacks after carbocation forms.
How does the substrate type influence whether nucleophile strength matters more?
Primary substrates almost always follow SN2 and need strong nucleophiles. Tertiary substrates usually react via SN1, where nucleophile strength matters less. Secondary substrates depend on both factors.
What solvents favor SN1 or SN2, and how does this relate to nucleophile strength?
Protic solvents stabilize carbocations, promoting SN1, making nucleophile strength less critical. Aprotic solvents favor SN2 by allowing strong nucleophiles to attack directly.
How are nucleophilicity and basicity related in these reactions?
Nucleophilicity and basicity often correlate. But nucleophiles react faster kinetically than bases, which reflect thermodynamic stability. This difference affects how they behave in SN2 reactions.



Leave a Comment