E1 or E2: Determining the Elimination Mechanism

The choice between E1 and E2 elimination mechanisms depends primarily on the substrate structure, the base strength and steric hindrance, the solvent type, and the reaction conditions. Understanding these variables helps predict which pathway a given reaction will follow.
Substrate Structure and Its Impact
The nature of the carbon bearing the leaving group heavily influences whether a reaction proceeds via E1 or E2.
- Tertiary Carbons Favor E1: Tertiary carbons stabilize carbocations well. This makes the two-step E1 mechanism feasible, as the rate-determining step involves carbocation formation. For example, tertiary alkyl halides often undergo E1 elimination since their carbocations are relatively stable.
- Primary Carbons Favor E2: Primary carbons form unstable carbocations. Thus, elimination typically proceeds via a concerted E2 mechanism, where the base removes a proton as the leaving group departs simultaneously. This is especially true in aprotic solvents with strong bases.
- Secondary Carbons: These can follow either pathway, depending on other factors like base strength and solvent.
Role of the Base
Base strength and steric factors play key roles in determining elimination pathways.
- Weak Bases Favor E1: E1 reactions commonly occur with weak bases because formation of the carbocation intermediate does not require a strong base for deprotonation. Weak bases such as water, alcohols, or sterically hindered amines often lead to E1.
- Strong and Bulky Bases Favor E2: Strong, hindered bases tend to promote E2 mechanisms by abstracting a proton in a single concerted step. Bulky bases often give the less substituted alkene (Hofmann product) due to steric hindrance preventing removal of more substituted protons.
- Amines and Their Role: Tertiary amines like trimethylamine (NMe3) or triethylamine (TEA) vary in their basicity and steric bulk. Trimethylamine is a weaker base and gaseous at room temperature, less likely to induce E2 exclusively. TEA, a liquid at room temperature, is more sterically hindered and a stronger base, often favoring E2.
Effect of Solvent

Solvent effects distinguish E1 and E2 further.
- Protic Solvents Favor E1: Protic solvents stabilize carbocation intermediates via hydrogen bonding, encouraging the two-step E1 mechanism.
- Aprotic Solvents Favor E2: Aprotic solvents do not stabilize ions well, thus favoring the concerted E2 pathway. They are typically used with strong bases in elimination reactions.
- Unspecified Solvent Conditions: When solvent identity is unknown, predicting the mechanism is challenging. However, assumptions based on common solvents help guide expectations.
Product Distribution: Hoffman vs Zaitsev
Elimination products can indicate the underlying mechanism:
| Mechanism | Typical Product | Product Characteristics |
|---|---|---|
| E2 | Hofmann Product (less substituted alkene) | Bulky bases favor elimination at the less hindered β-hydrogen, yielding less substituted alkenes. |
| E1 | Zaitsev Product (more substituted, thermodynamic alkene) | Carbocation intermediates rearrange, often yielding the more substituted, stable alkene. |
Although generally reliable, product distribution can vary based on reaction conditions. Some E2 reactions also produce Zaitsev products, depending on base and substrate.
Reaction Pathway Dynamics
Multiple pathways compete during elimination:
- SN vs E Mechanisms: Tertiary substrates with bulky bases and good leaving groups seldom undergo SN2 due to steric hindrance. Instead, elimination (E1 or E2) is preferred.
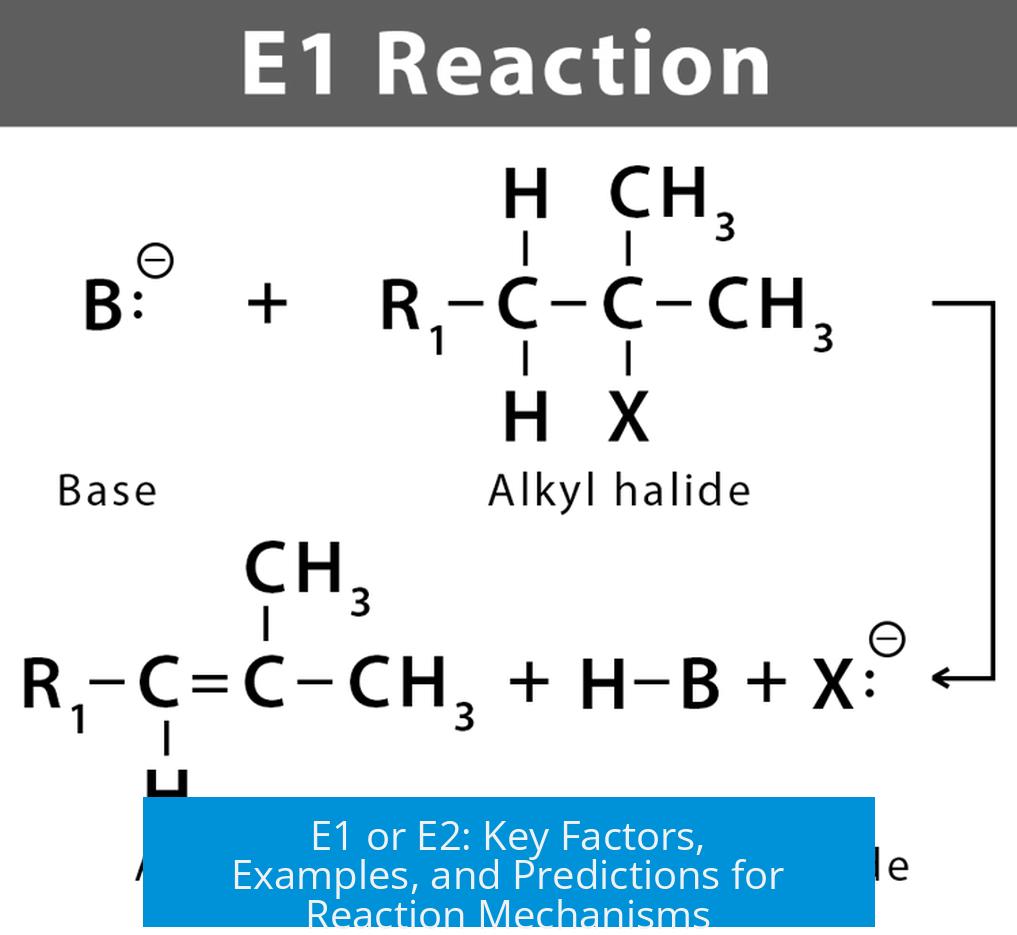
- Carbocation Intermediate: E1 proceeds via carbocation formation after the leaving group departs. The intermediate then loses a proton to form the double bond.
- E2 Concerted Process: E2 occurs in one step where the base abstracts a proton as the leaving group leaves simultaneously, without carbocation formation.
Influence of Temperature and Other Conditions
Temperature often dictates elimination vs substitution but was unspecified here.
- Higher temperature typically favors elimination.
- Without data on temperature or solvent, predictions rest on substrate and base information.
Summary of Key Factors Differentiating E1 and E2
- Substrate: Tertiary carbons favor E1; primary favor E2.
- Base: Weak, small bases incline toward E1; strong, bulky bases favor E2.
- Solvent: Protic solvents support E1 by stabilizing carbocations; aprotic solvents encourage E2.
- Product: E1 usually yields Zaitsev (more substituted) alkenes; E2 with bulky bases yields Hofmann (less substituted) products.
- Mechanistic Pathways: E1 via carbocation intermediate; E2 is a concerted mechanism.
Practical Examples
Consider elimination from a tertiary alkyl bromide using trimethylamine:
- Trimethylamine is a weak, somewhat hindered base and a gas at room temperature.
- The tertiary carbon supports carbocation formation.
- In protic solvent, E1 is likely as weak base and stable carbocation favor this.
- However, if an aprotic solvent is present, E2 may compete due to solvent effects assisting a concerted elimination.
In contrast, triethylamine, a stronger base with steric hindrance, increases E2 likelihood, often producing Hofmann products, especially with primary or secondary carbons.
Conclusion: Predicting E1 or E2
Distinguishing E1 from E2 elimination requires integrating information about the substrate, base, solvent, and reaction conditions. Both mechanisms compete, but careful analysis helps identify the predominant pathway.
Key Takeaways:
- Tertiary substrates and weak bases generally lead to E1 due to carbocation stability.
- Strong, bulky bases favor E2 by concerted proton abstraction and leaving group departure.
- Protic solvents stabilize carbocations, supporting E1; aprotic solvents favor E2.
- Product distribution (Hofmann vs Zaitsev) offers clues about the mechanism.
- Absence of solvent and temperature data complicates definitive assignment.
What role does substrate structure play in deciding between E1 and E2?
Tertiary carbons favor E1 due to stable carbocation formation. Primary carbons lean toward E2, especially with aprotic solvents. Steric hindrance also affects which elimination pathway is preferred.
How does solvent type influence whether E1 or E2 will occur?
Protic solvents tend to favor E1 reactions by stabilizing carbocations. Aprotic solvents promote E2 by allowing strong base deprotonation without stabilizing carbocations. Solvent choice is crucial but often unspecified.
Why do weak bases favor E1 while strong or bulky bases favor E2?
Weak bases cannot easily remove protons, so the reaction proceeds via E1 with carbocation formation. Strong or bulky bases abstract protons faster, pushing elimination through E2 and often yielding the Hoffmann product.
How does base steric hindrance affect the product distribution in elimination?
Bulky bases favor the Hoffmann product (less substituted alkene) in E2 reactions. Smaller or weaker bases often lead to thermodynamic, more substituted products typical of E1 pathways.
What distinguishes the E1 elimination mechanism from E2 in terms of intermediates?
E1 involves carbocation intermediates formed after the leaving group departs, allowing rearrangement. E2 is a concerted reaction where proton removal and leaving group departure happen simultaneously, with no intermediate.
Can both E1 and E2 happen under the same conditions?
Yes, E1 and E2 can compete depending on substrate, base strength, solvent, and temperature. For example, a tertiary carbon with a weak base in an aprotic solvent can show characteristics of both mechanisms.



Leave a Comment