E1 vs E2 Reactions: Understanding Zaitsev and Hoffmann Products

The key difference in product selectivity between E1 and E2 elimination reactions lies in their regioselectivity and mechanistic constraints. E1 generally favors the Zaitsev product but offers limited regiochemical control, while E2 commonly yields the Zaitsev product unless influenced by bulky bases or substrate geometry, which can favor the Hoffmann product.
Regiochemical Outcome of E1 and E2

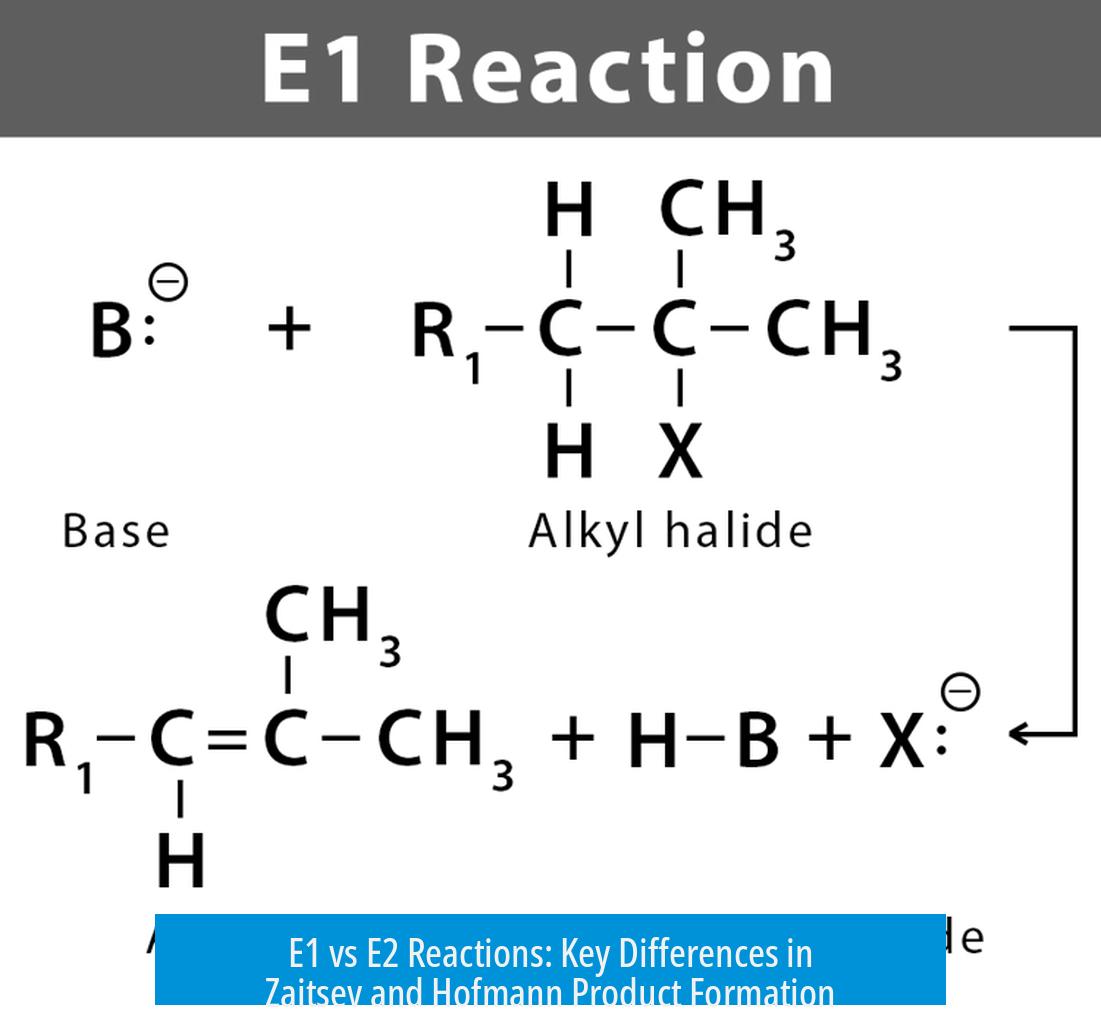
- E1 Reaction: E1 elimination typically produces the more substituted alkene, known as the Zaitsev product, as the major product. However, this reaction pathway does not guarantee exclusive formation of this product. Side products may form due to carbocation rearrangements and limited regiochemical control.
- E2 Reaction: E2 elimination usually favors the Zaitsev product under normal conditions. The reaction proceeds via a concerted mechanism, allowing regiochemical control. However, bulky or sterically hindered bases promote the formation of the Hoffmann product, which is the less substituted alkene. Additionally, alkyl fluorides often give the Hoffmann product due to their unique properties.
E2 Mechanistic and Stereochemical Constraints
E2 elimination requires a strict anti-coplanar geometry between the β-hydrogen and the leaving group for the reaction to proceed. This stereochemical necessity can influence which β-hydrogen is eliminated, occasionally directing the process toward the Hoffmann product. Strong bases are essential in E2 to abstract the β-hydrogen effectively and drive the reaction to completion.
Factors Influencing E1 vs E2 and Product Distribution
The outcomes of elimination reactions depend strongly on external factors such as the nature of the base, solvent, and temperature:
- Base Strength and Steric Hindrance: Strong, non-bulky bases favor E2 and the Zaitsev product; bulky bases favor the Hoffmann product by steric hindrance.
- Solvent: Polar protic solvents stabilize carbocations, favoring E1; polar aprotic solvents favor E2 pathways.
- Temperature: Higher temperatures generally promote elimination over substitution.
Summary of Key Points
- E1 elimination primarily yields the Zaitsev product but lacks full regiochemical control.
- E2 elimination favors the Zaitsev product unless sterically hindered bases or substrate geometry promotes the Hoffmann product.
- E2 requires an anti-coplanar configuration between the β-hydrogen and leaving group, influencing product formation.
- Base strength, steric bulk, solvent type, and temperature significantly affect elimination type and product distribution.
What determines whether E1 or E2 elimination favors the Zaitsev or Hoffmann product?
E1 usually gives mostly the Zaitsev product but lacks strict control. E2 generally favors Zaitsev unless a bulky base or alkyl fluoride is present, which shifts preference to the Hoffmann product by steric or electronic effects.
Why does E2 elimination sometimes produce the Hoffmann product instead of Zaitsev?
E2 requires the β-hydrogen and leaving group to be anti-coplanar. This geometric need can prevent formation of the more substituted alkene, leading to the less substituted Hoffmann product, especially with bulky bases.
How do bases affect the regiochemical outcome in E2 elimination?
- Strong, small bases favor the Zaitsev product by removing the most substituted β-hydrogen.
- Bulky bases hinder access to substituted sites, pushing elimination to less substituted β-hydrogens, producing the Hoffmann product.
Can solvent or temperature influence whether elimination follows E1 or E2 pathways?
Yes, polar protic solvents and higher temperatures often favor E1. Strong bases, polar aprotic solvents, and moderate to high temperature usually steer the reaction to E2, impacting which alkene forms.
Does E1 always give a single elimination product?
No. E1 typically produces the Zaitsev product as the major one, but multiple alkene isomers can form because the reaction lacks tight regiochemical control.




Leave a Comment