Electrophilic vs Nucleophilic: Understanding the Core Differences
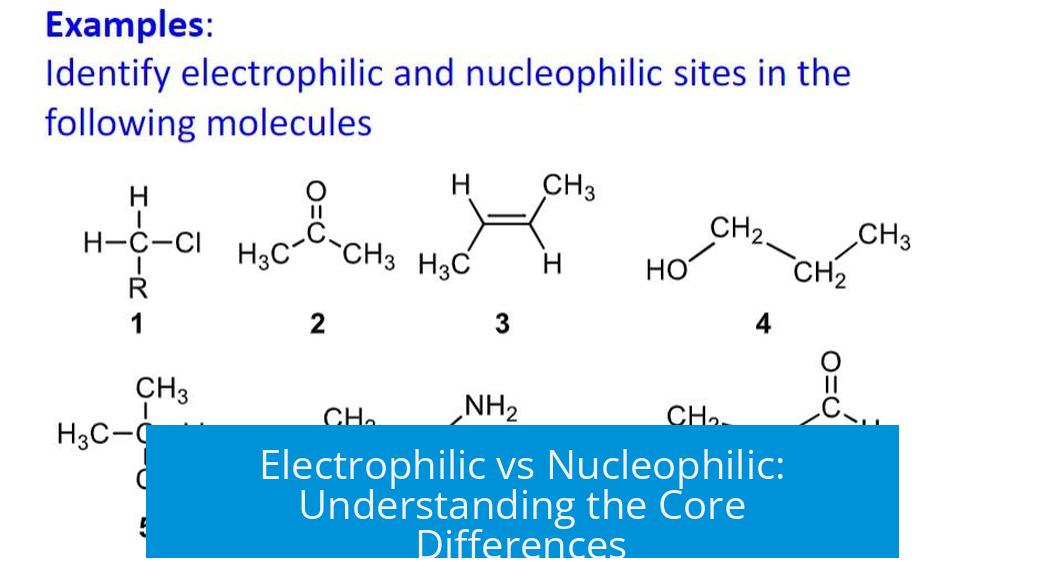
Electrophilic and nucleophilic species differ primarily in their electron density and the nature of their charge interactions, with nucleophiles being electron-rich and attracted to positive charges, while electrophiles are electron-deficient and attracted to electrons or negatively charged species. This fundamental distinction governs their behavior in chemical reactions.
What is a Nucleophile?
A nucleophile is a chemical species that is attracted to a positive charge or a nucleus. It is electron-rich, often carrying a negative charge, which makes it prone to donate electrons or accept protons.
- Common examples include conjugate bases with negative charges.
- The methyl anion (CH3−) is a stronger nucleophile than the amide ion (NH2−), as it has a higher pKa and a stronger desire to accept a proton.
- Resonance reduces nucleophilicity because the negative charge is spread out, stabilizing the molecule and diminishing its tendency to donate electrons.
In other words, nucleophilicity relies heavily on localized high electron density. Steric hindrance, or the presence of bulky groups around the nucleophilic center, can block access and reduce its reactivity.
What is an Electrophile?
An electrophile is a species attracted to electrons, often positively charged or electron-deficient. These molecules seek to react with electron-rich species to gain electrons and stabilize their charge.
- Examples include carbocations (C+), which carry an unstable positive charge and actively seek electrons.
- The electrophilicity of a molecule depends on its stability and its tendency to accept electrons or protons.
- Electrophiles generally have low electron density, making them vulnerable to attack by nucleophiles.
Electrophiles may also experience steric hindrance, which can affect their reactivity by blocking access to the positive center.
Meaning of the Terms: ‘Phile’, ‘Electro’, and ‘Nucleo’
The prefixes and suffixes help clarify the types of chemical affinity these species express.
- ‘Phile’ means “lover” or “attracted to.”
- ‘Electro’ signifies “electric charge,” often positive.
- ‘Nucleo’ refers to “nucleus,” indicating attraction toward positive centers or nuclei.
Thus, a nucleophile is a “nucleus lover,” attracted to regions of positive charge, while an electrophile is an “electron lover,” attracted to regions rich in electrons.
Electron Density: The Core Concept
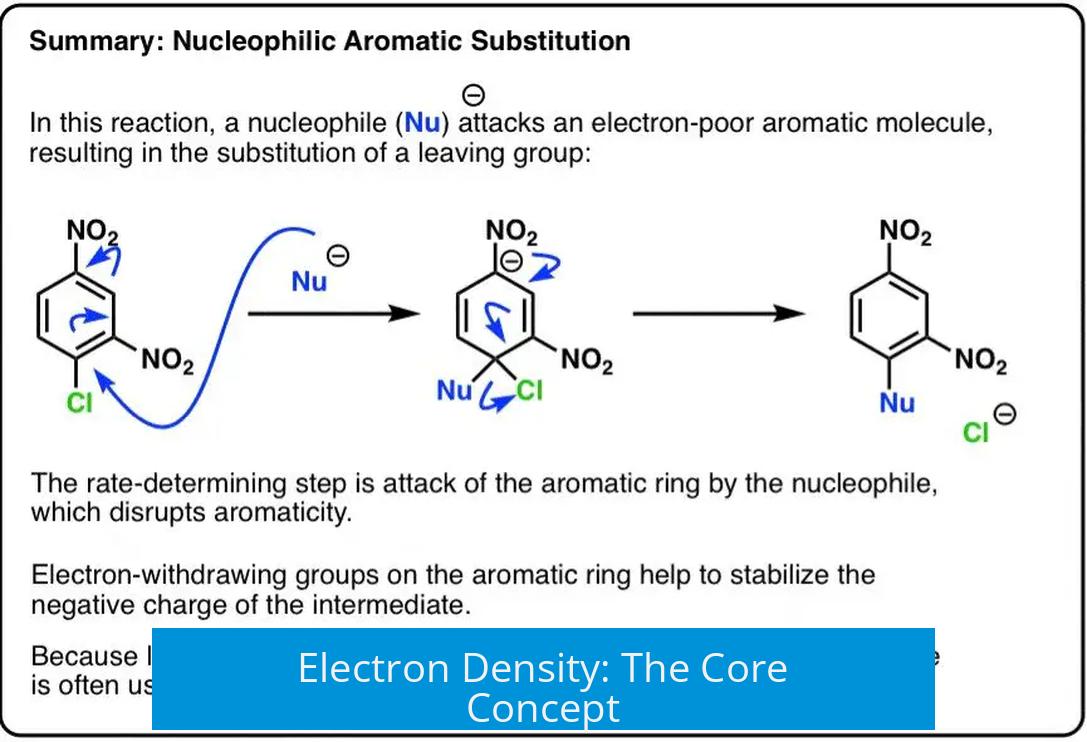
The distinction between nucleophiles and electrophiles fundamentally depends on electron density:
| Property | Nucleophiles | Electrophiles |
|---|---|---|
| Electron Density | High electron density localized on the atom or molecule | Low electron density; electron-poor species |
| Charge | Often negatively charged or neutral but electron-rich | Often positively charged or neutral but electron-deficient |
| Reactivity Influence | Steric hindrance reduces access and lowers nucleophilicity | Steric hindrance can impede access, reducing electrophilicity |
| Stability Effect | Resonance delocalization decreases nucleophilicity by stabilizing charge | Less stable positive species tend to be more electrophilic |
Resonance Effects on Nucleophilicity
Molecules stabilized by resonance show decreased nucleophilicity. This is because the negative charge is spread over several atoms, reducing its reactivity and electron-donating ability.
For example, the acetate ion (CH3COO−) is less nucleophilic than the methoxide ion (CH3O−) due to resonance stabilization in acetate.
Examples Illustrating the Concepts
- Nucleophiles: Hydroxide ion (OH−), alkoxide ions, amines, and halide ions (Cl−, Br−).
- Electrophiles: Carbocations, carbonyl carbons, proton (H+), alkyl halides during substitution reactions.
Reactivity depends not only on charge but on surrounding atoms and molecular structure. Electron-withdrawing groups nearby enhance electrophilicity by decreasing electron density. Electron-donating groups increase nucleophilicity.
Summary Table of Electrophilic vs Nucleophilic Characteristics
| Aspect | Nucleophilic Species | Electrophilic Species |
|---|---|---|
| Charge Preference | Attracted to positive centers (nucleus) | Attracted to electrons or negative regions |
| Typical Charge | Negative or electron-rich | Positive or electron-poor |
| Effect of Resonance | Reduces nucleophilicity by charge delocalization | Varies based on electrophile structure |
| Role of Steric Hindrance | Blocks access to electron-rich site; lowers nucleophilicity | Can obstruct positive centers; affects electrophilicity |
Key Takeaways
- Nucleophiles are electron-rich species attracted to positive charges and nuclei.
- Electrophiles are electron-poor or positively charged and attracted to electrons.
- Resonance delocalizes charge, reducing nucleophilicity by stabilizing negative charge.
- Steric hindrance diminishes nucleophilicity by preventing access to electron dense sites.
- Understanding prefixes like “phile” helps clarify the nature of attraction in these species.
What defines a nucleophile in chemical reactions?
A nucleophile is attracted to positive charges or nuclei. It has high electron density and tends to donate electrons to electrophiles. Conjugate bases like CH3⁻ are common nucleophiles because they strongly seek protons.
How does resonance affect nucleophilicity?
Resonance spreads out negative charge across a molecule. This charge delocalization stabilizes the molecule, which lowers its nucleophilicity. So, molecules with resonance are less nucleophilic than those with concentrated charge.
What characterizes an electrophile compared to a nucleophile?
Electrophiles have low electron density and carry positive charge or partial positive charge. They seek electrons and react with electron-rich nucleophiles. Carbocations (C⁺) are typical electrophiles eager to neutralize their positive charge.
Why does steric hindrance reduce nucleophilicity?
Steric hindrance blocks access to the electron-rich area of a nucleophile. This prevents reaction with electrophiles. So, bulky groups near the nucleophilic site lower its ability to donate electrons effectively.
How do you determine if a species is more nucleophilic or electrophilic?
Look at electron density and stability. High electron density favors nucleophilicity; low electron density and positive charge indicate electrophilicity. Also, species wanting to accept or donate electrons help identify their type.





Leave a Comment