Understanding Enantiomers vs Diastereomers
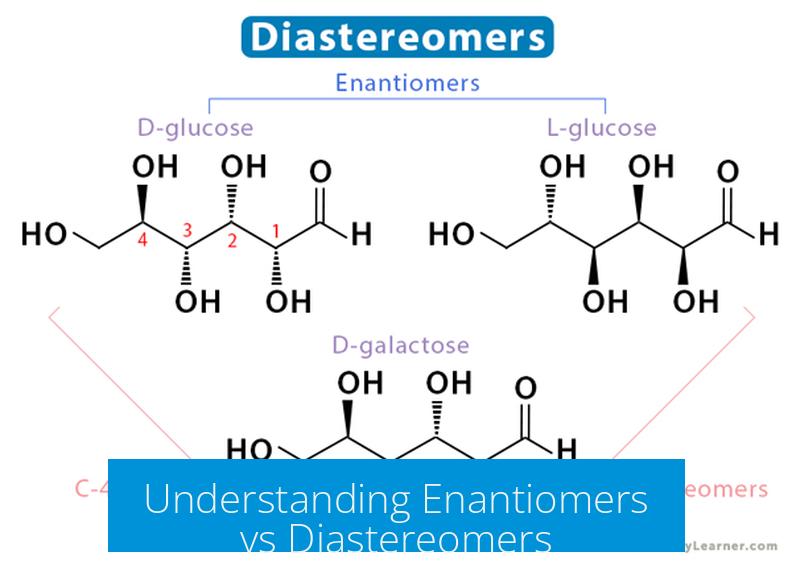
Enantiomers and diastereomers are types of stereoisomers distinguished by their spatial arrangements around chiral centers. Both play critical roles in stereochemistry and influence the physical and chemical properties of molecules.
Definitions and Key Characteristics
Enantiomers
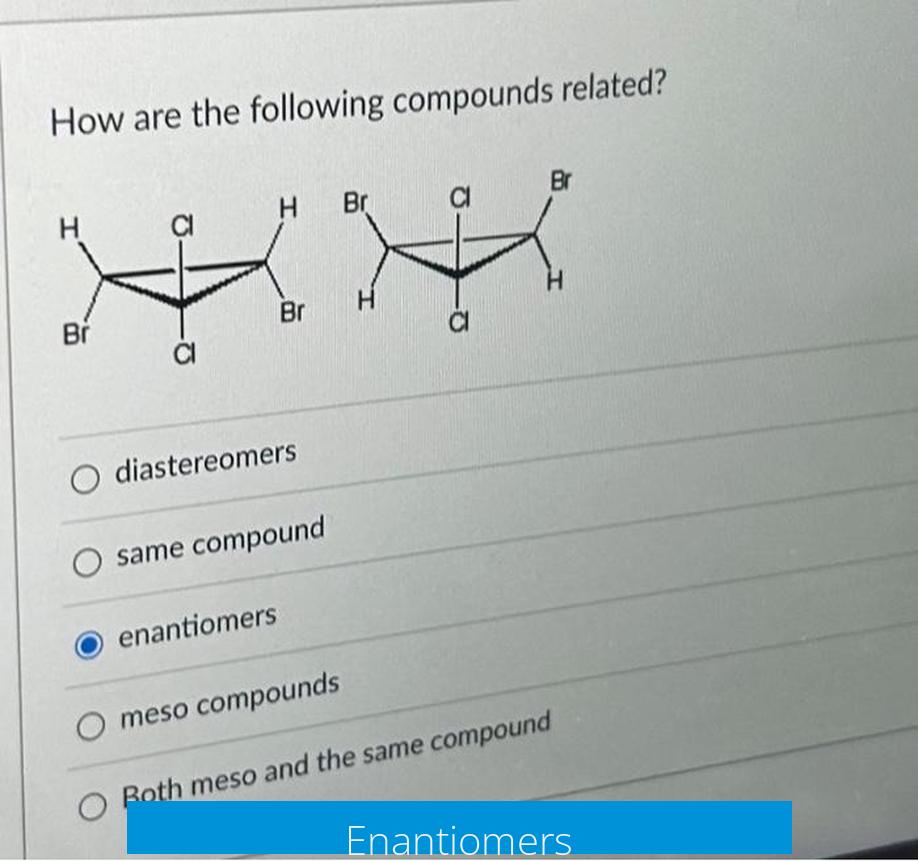
Enantiomers are pairs of compounds that are non-superimposable mirror images of each other. They contain chiral centers with opposite configurations at every stereocenter. This mirror-image relationship means one enantiomer is the exact spatial opposite of the other.
Diastereomers
Diastereomers are stereoisomers that have at least two chiral centers, but differ in the configuration at one or more (not all) stereocenters. They are not mirror images and cannot be superimposed. As a result, diastereomers often possess different physical and chemical properties.
Examples to Clarify
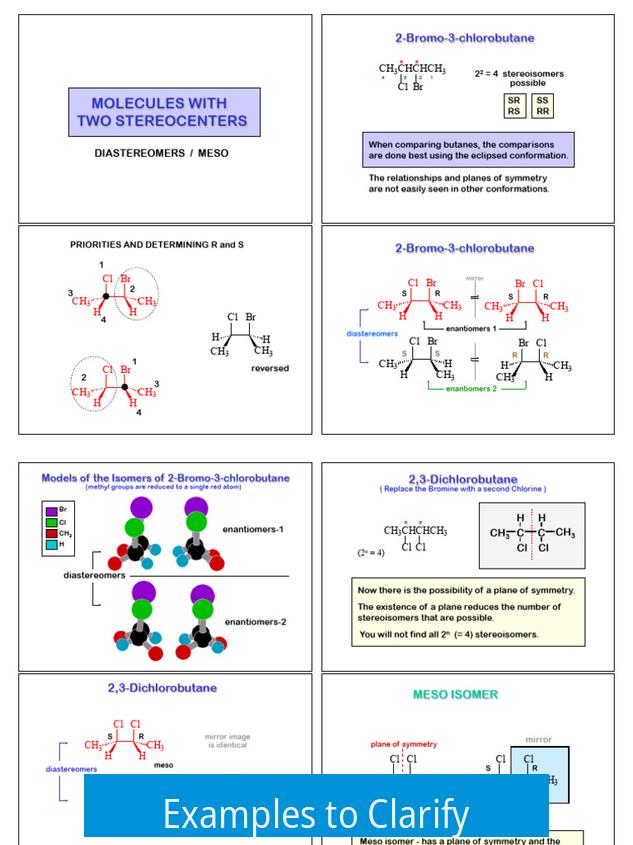
| Type | Example Pair | Description |
|---|---|---|
| Enantiomers | R,R and S,S | All chiral centers inverted; mirror images |
| Diastereomers | R,R and R,S | One chiral center inverted; not mirror images |
Further examples include:
- R,R,R and S,S,S are enantiomers — every chiral center is inverted.
- R,R,S and R,S,S are diastereomers — some but not all centers differ.
Summary of Differences
- Enantiomers differ at all chiral centers; diastereomers differ at some but not all centers.
- Enantiomers are mirror images; diastereomers are not.
- Diastereomers can exhibit distinct chemical and physical traits, unlike enantiomers which are mostly identical except for optical activity.
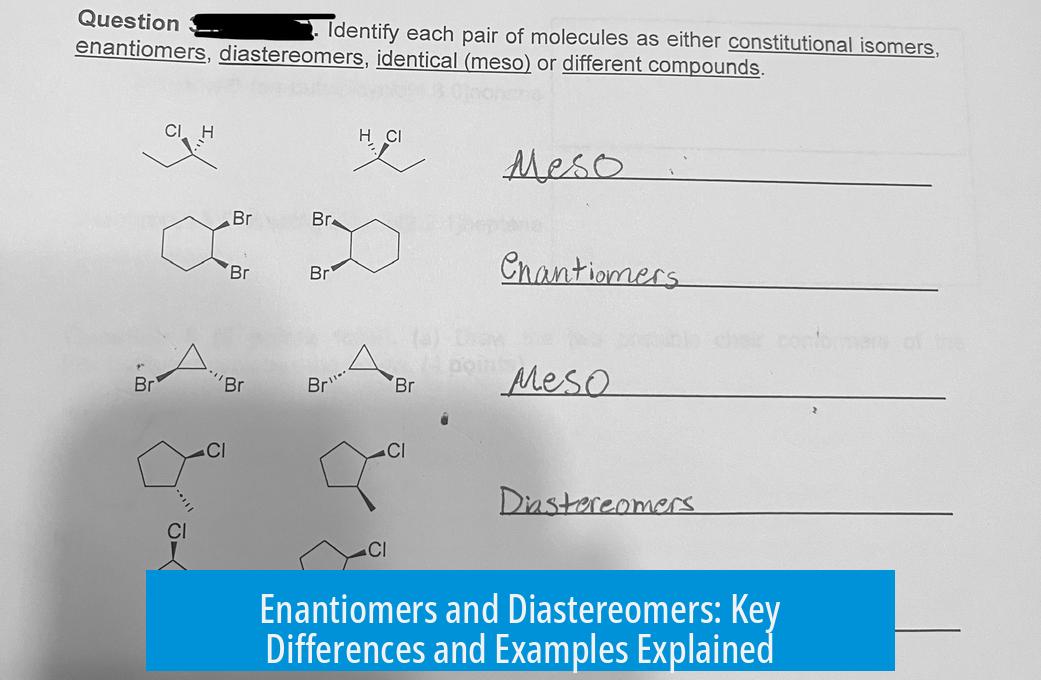
An effective visual representation illustrating these concepts can be found here.
Key Takeaways
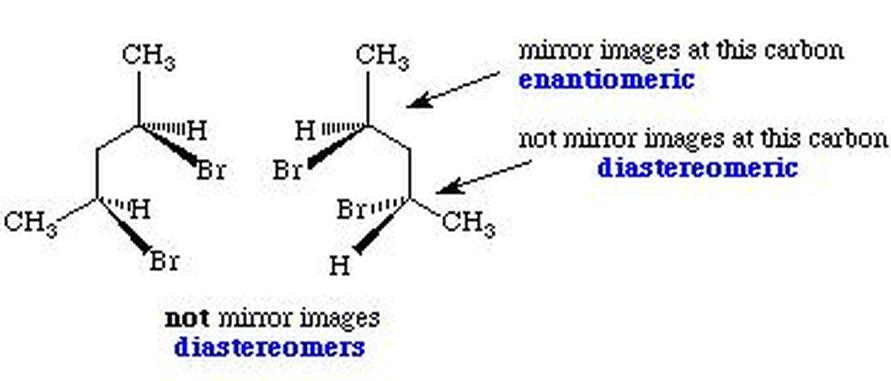
- Enantiomers are mirror-image stereoisomers differing at every chiral center.
- Diastereomers differ at some but not all chiral centers and are not mirror images.
- Diastereomers exhibit distinct chemical and physical properties; enantiomers usually do not.
- Understanding these differences is essential for applications in chemistry and pharmaceuticals.
Enantiomers vs Diastereomers: What’s the Real Difference?
Enantiomers and diastereomers are both types of stereoisomers, but they have distinct differences that chemists absolutely love to debate about. Simply put, enantiomers are mirror-image molecules that can’t be superimposed, while diastereomers are stereoisomers that aren’t mirror images at all. If you’ve ever scratched your head trying to untangle these concepts, you’re not alone. Let’s dive deep with clear examples, intriguing twists, and practical understanding.
Have you ever tried to fit your left glove onto your right hand? That’s basically what enantiomers are like—mirror images that just won’t line up. But what about those cousins that look similar but aren’t exactly mirror images and definitely don’t fit like gloves? Those are diastereomers. Ready to unravel this stereochemical mystery?
What Exactly Are Enantiomers?
Enantiomers are molecules containing one or more chiral centers. These chiral centers are carbon atoms connected to four different groups. The magic happens when you take a molecule and create its mirror image. If this mirror image cannot be exactly placed on top of the original in 3D space, you’ve got yourself a pair of enantiomers.
For example, take an object with two chiral centers configured as R,R. Its enantiomer mirrors all chiral centers, becoming S,S. Think of them as perfect but non-overlapping mirror doubles. Chemically, they behave very similarly, but in biological systems, they might have vastly different effects—like left- and right-handed gloves fitting different hands.
What Makes Diastereomers Different?
Now, diastereomers are a bit more rebellious. They’re also stereoisomers but with a catch. Imagine two molecules with at least two chiral centers. If some—but not all—of these chiral centers are flipped, the molecules become diastereomers.
Unlike enantiomers, diastereomers are not mirror images of each other. This distinction gives diastereomers unique physical and chemical properties, unlike enantiomers that often share many traits. So, while enantiomers tend to be chemical doppelgängers, diastereomers march to their own beat.
For instance, consider the pairs R,R and R,S. Here, one chiral center flips but the other stays the same, making them diastereomers. The difference isn’t just in theory—this can mean variations in boiling points, solubility, and even chemical reactivity. If enantiomers are the twin siblings, diastereomers might be cousins with noticeably different personalities.
More Complex Examples: Because Chemistry Loves to Confuse Us
Okay, here’s where it gets a bit trickier—molecules with three chiral centers. Let’s say we have two compounds: R,R,R and S,S,S. These are enantiomers again—the exact mirror images with all chiral centers inverted.
But what about R,R,S and R,S,S? They differ at only one center, so they’re diastereomers. Notice a pattern? The rule is simple but crucial:
- If all chiral centers are inverted in one molecule compared to another, they are enantiomers.
- If only some chiral centers flip, the molecules are diastereomers.
This tiny tweak can cause huge changes. Sometimes, diastereomers exhibit very different smells, tastes, or drug potencies, whereas enantiomers may closely mirror each other except in polarized light rotation.
Why Care About These Differences?
Understanding these concepts isn’t just a fun puzzle. It’s critical for practical fields like drug development. Some medications work perfectly as one enantiomer but prove ineffective or even harmful as the other. Knowing whether you’re dealing with enantiomers or diastereomers determines how you deploy chemical synthesis, purification, and testing.
Also, the physical differences between diastereomers often allow easier separation. Unlike enantiomers, which sometimes need special techniques to isolate, diastereomers can often be separated with regular chromatography or recrystallization.
Visual Aid: Seeing Is Believing
Sometimes, words just won’t cut it. This helpful image (click here to view) brilliantly illustrates the difference between enantiomers and diastereomers. It shows how flipping different chiral centers impacts molecule symmetry and relationship.
Visual learning can cement these concepts far better than text alone. If you’re tackling organic chemistry, I highly recommend referencing this graphic.
The Bottom Line
Enantiomers are non-superimposable mirror images differing in configuration at every chiral center, while diastereomers differ at some but not all chiral centers and are not mirror images. This difference affects their physical and chemical behavior, making it vital for chemists, pharmacists, and researchers to distinguish between them. It’s not just academic nitpicking; it’s about understanding how molecules work in the real world.
If you’re ever stuck between these terms during your studies or career, just ask yourself:
- Are my two molecules mirror images with all chiral centers inverted? If yes, enantiomers.
- Do they differ at some but not all chiral centers and aren’t mirror images? That’s diastereomers.
Simple questions, big clarity. Now, go forth and conquer chirality with confidence!




Leave a Comment