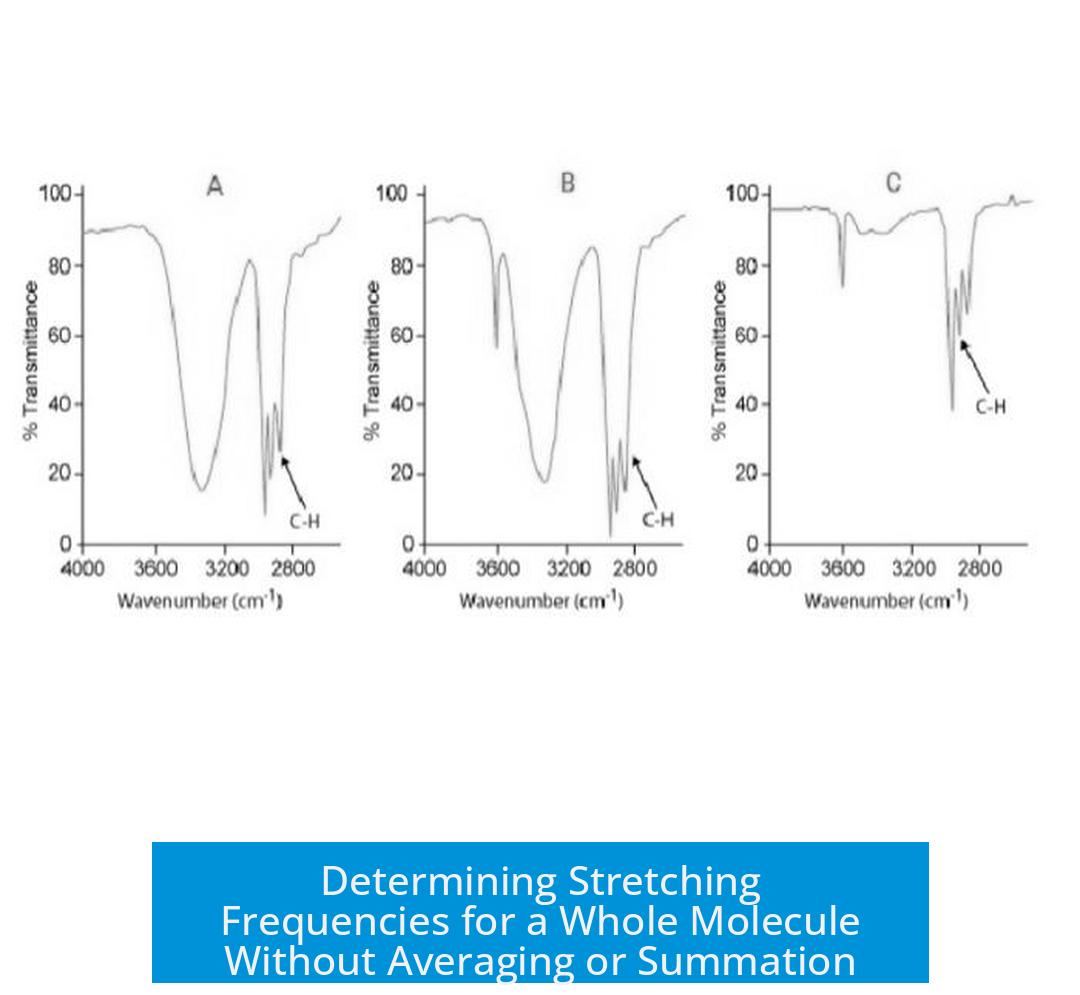
Determining Stretching Frequencies for a Whole Molecule
You do not determine a single stretching frequency for a whole molecule by averaging or summing individual stretches. Instead, each functional group in a molecule has its own characteristic stretching frequency, and these coexist independently in the molecule’s IR spectrum. Knowing this is crucial for interpreting or predicting vibrational spectra accurately.
Stretching Frequencies Arise from Functional Groups
A molecule’s vibrational spectrum shows multiple bands corresponding to different parts of the molecule. For example:
- Carbonyl groups (C=O) typically absorb near 1700 cm−1
- Benzene rings show characteristic C=C stretches around 1600 cm−1
- sp2 C-H stretches appear near 3000 cm−1
Each type of bond stretch occurs at a specific frequency range based on bond strength and atomic masses involved.
No Averaging or Summation of Frequencies
Stretching vibrations are not averaged or summed across the molecule. Instead, the IR spectrum shows all relevant bands from each functional group simultaneously. This means the spectrum is a composite of distinct signals, not a single unified frequency.
Estimating and Identifying Frequencies Practically
To determine stretching frequencies in a molecule, first:
- Identify all key functional groups present.
- Consult reference tables or spectra showing typical absorption ranges for these groups.
- Match predicted vibrations to those ranges rather than trying to combine frequencies.
This approach aligns with standard IR interpretation practices in organic chemistry and analytical methods.
Handling Common Confusions
Sometimes exam or textbook questions incorrectly imply a single frequency for an entire molecule or offer misleading options. These issues can cause confusion, but understanding the nature of vibrational spectroscopy helps avoid errors.
Experienced students learn to disregard such flawed question constructions and focus on functional group frequencies individually.
Key Takeaways
- The whole molecule does not have one average stretching frequency.
- Each functional group contributes its own distinct stretch frequency.
- Stretching frequencies coexist independently in the IR spectrum.
- Identify stretches by functional group absorption ranges, not by averaging.
- Critically evaluate flawed questions and focus on functional group analysis.
How do I determine stretching frequencies for an entire molecule?
Look at each functional group individually. Identify where typical stretches like C=O or aromatic C-H appear. The molecule does not have a single combined frequency; each group shows its own distinct absorption.
Is the overall stretching frequency of a molecule an average of all functional groups?
No, the frequencies are not averaged. Each functional group contributes separate stretching frequencies. They coexist independently in the IR spectrum without combining into one value.
How can I estimate stretching frequencies for unknown molecules?
Break the molecule into functional groups. Then use reference data for each group’s known absorption ranges. This approach lets you predict where each stretch should appear in the spectrum.
Why don’t we just sum or average the stretching frequencies?
Because the molecular vibrations happen independently, summing or averaging would lose specific information about each bond. The IR spectrum shows distinct peaks for each bond type without merging them.
What should I focus on when answering questions about molecular stretching frequencies?
Focus on identifying the functional groups and their typical absorption frequencies. Avoid expecting a single frequency or an average. Pay attention to each stretch separately for accurate interpretation.


Leave a Comment