How to Tell Which H Atom Is More Acidic and Why
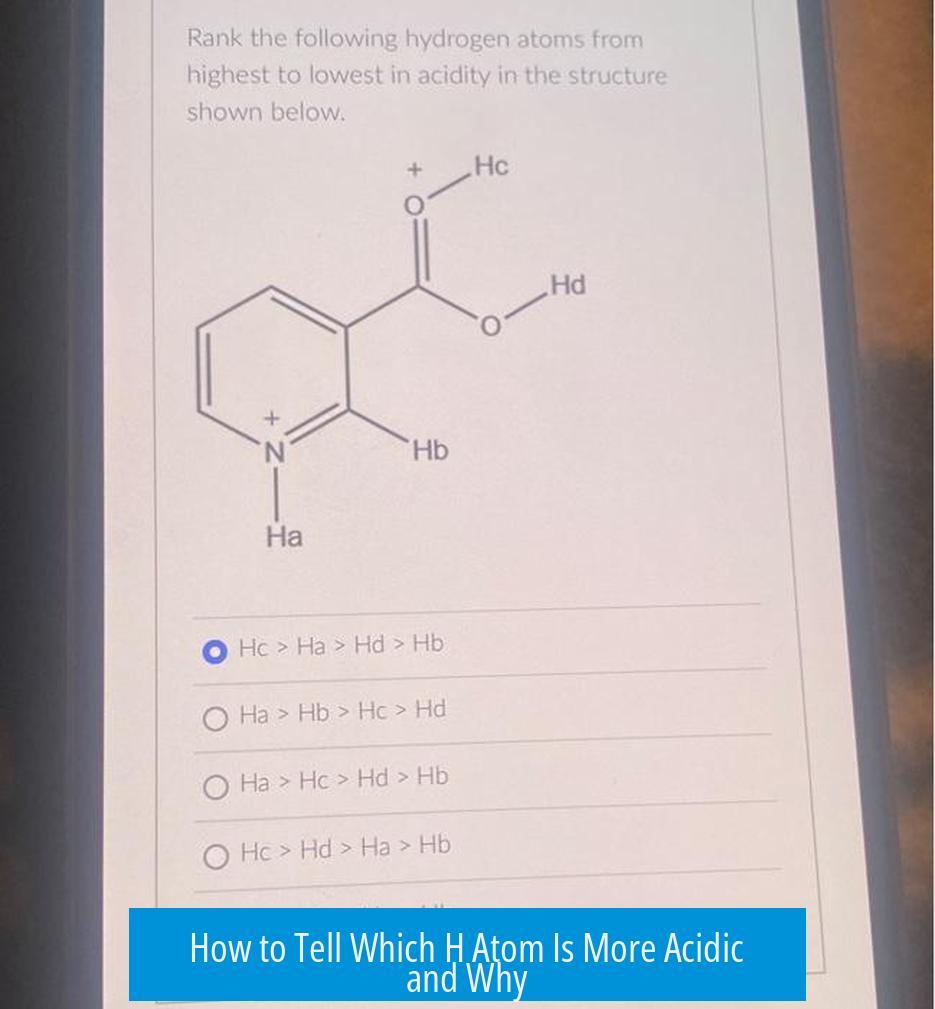
The acidity of a hydrogen (H) atom depends on the stability of its conjugate base after deprotonation; the more stable the conjugate base, the more acidic the H atom. This stability arises from factors such as resonance delocalization, electronegativity of the atom bearing the negative charge, inductive effects, and orbital hybridization. By analyzing these characteristics, one can predict which proton in a molecule will be most acidic.
1. Initial Screening of Protons
Start by identifying which hydrogens are attached to atoms capable of stabilizing the negative charge after losing a proton. Hydrogens bonded to carbons without resonance or electronegative neighbors tend not to be acidic.
- Exclude hydrogens attached to alkyl carbons.
- Focus on hydrogens attached to heteroatoms like oxygen, nitrogen, or carbon atoms adjacent to electron-withdrawing groups.
- Consider hydrogens attached to atoms capable of resonance stabilization of their conjugate bases.
For example, in a molecule with six distinct protons, often four can be excluded quickly because their conjugate bases would not be stable.
2. Stability of the Conjugate Base Is the Key Indicator
Acidity depends primarily on how stable the conjugate base becomes once the proton is removed. Draw the conjugate bases for each candidate proton by removing the proton and assigning the negative charge appropriately.
- More resonance forms that delocalize the negative charge imply a more stable conjugate base.
- Charge delocalization to electronegative atoms such as oxygen increases stability.
- Less resonance or poorly delocalized charges mean lower acidity.
For example, a proton whose conjugate base can delocalize its negative charge onto a nearby carbonyl group is more acidic than one lacking such resonance.
3. Drawing Conjugate Bases and Applying Core Concepts
Once conjugate bases are drawn, analyze them using these organic chemistry principles:
- Electronegativity: Negative charge on a more electronegative atom is more stable.
- Resonance: Greater resonance delocalization enhances stability.
- Inductive effects: Nearby electron-withdrawing or donating groups influence charge stability through polarization.
- Orbital hybridization: Negative charge on an sp-hybridized atom is more stable than sp2, which is more stable than sp3.
This process narrows down which proton is more acidic.
4. Use ARIO Framework for Systematic Analysis
ARIO is a well-known acronym to evaluate acidity through conjugate base stability:
| Factor | Description |
|---|---|
| Charge | Less concentrated negative charge (closer to neutral) increases stability. |
| Atom | Stability depends on the electronegativity and size of the atom bearing the negative charge. |
| Resonance | Delocalization of negative charge over multiple atoms increases stability. |
| Induction | Electron-withdrawing groups stabilize negative charge via polarization. |
| Orbitals | Hybridization matters: sp (most acidic) > sp2 > sp3 (least acidic) in charge stability. |
Applying ARIO to the conjugate base helps predict relative acidities.
5. Factors Affecting Acidity
Atomic Factors
- Electronegativity: More electronegative atoms better stabilize negative charge.
- Polarizability: Larger atoms spread charge over bigger volume, increasing stability.
- Hybridization: Negative charge in an orbital with more s-character (sp) is held closer to the nucleus and is more stable.
Environmental Factors
- Electron Withdrawing/Donating Groups: Groups that withdraw electrons via induction increase acidity; donating groups decrease it.
- Resonance Structures: Effective resonance stabilizing the conjugate base enhances acidity.
Resonance quality outweighs quantity. The extent to which resonance contributors stabilize the charge matters more than the number of resonance forms.
6. Practical Example: Acidic Hydroxyl Groups
Consider two different hydroxyl (-OH) groups within a molecule. The one whose conjugate base can delocalize the negative charge through resonance is more acidic. For example:
- The left hydroxyl group forms conjugate bases with resonance stabilization including a carbonyl group.
- The right hydroxyl group’s conjugate base lacks resonance stabilization.
- Hence, the left hydroxyl proton is more acidic.
Other hydrogens in the molecule, lacking these stabilizing effects, are substantially less acidic.
7. The Underlying Principle in Organic Chemistry Acidity
Organic acidity ultimately depends on conjugate base stabilization after proton loss. This fundamental rule applies universally to acidity, nucleophilicity, and reactivity in organic chemistry.
Familiarity with conjugate base structures and their stability guides understanding and prediction of acidity trends across diverse molecules.
8. Additional Resources and Examples
Examine molecules like ascorbic acid, which exhibit multiple hydroxyl groups with different acidities based on resonance and inductive effects. Such examples help reinforce principles discussed here.
Key Takeaways
- Acidity is linked to the stability of the conjugate base.
- Resonance delocalization largely dictates conjugate base stability.
- Electronegativity and orbital hybridization affect where the negative charge is best held.
- Inductive effects from neighboring groups can increase or decrease acidity.
- Drawing conjugate bases and applying the ARIO framework helps identify the most acidic proton.




Leave a Comment